Synthesis and HDAC inhibitory activity of pyrimidine-based hydroxamic acids
- PMID: 35923158
- PMCID: PMC9296983
- DOI: 10.3762/bjoc.18.84
Synthesis and HDAC inhibitory activity of pyrimidine-based hydroxamic acids
Abstract
Histone deacetylases (HDACs) play an essential role in the transcriptional regulation of cells through the deacetylation of nuclear histone and non-histone proteins and are promising therapeutic targets for the treatment of various diseases. Here, the synthesis of new compounds in which a hydroxamic acid residue is attached to differently substituted pyrimidine rings via a methylene group bridge of varying length as potential HDAC inhibitors is described. The target compounds were obtained by alkylation of 2-(alkylthio)pyrimidin-4(3H)-ones with ethyl 2-bromoethanoate, ethyl 4-bromobutanoate, or methyl 6-bromohexanoate followed by aminolysis of the obtained esters with hydroxylamine. Oxidation of the 2-methylthio group to the methylsulfonyl group and following treatment with amines resulted in the formation of the corresponding 2-amino-substituted derivatives, the ester group of which reacted with hydroxylamine to give the corresponding hydroxamic acids. The synthesized hydroxamic acids were tested as inhibitors of the HDAC4 and HDAC8 isoforms. Among the synthesized pyrimidine-based hydroxamic acids N-hydroxy-6-[6-methyl-2-(methylthio)-5-propylpyrimidin-4-yloxy]hexanamide was found to be the most potent inhibitor of both the HDAC4 and HDAC8 isoforms, with an IC50 of 16.6 µM and 1.2 µM, respectively.
Keywords: HDAC inhibitors; alkylation; aminolysis; hydroxamic acid; pyrimidine.
Copyright © 2022, Jakubkiene et al.
Figures
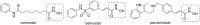


Similar articles
-
Synthesis and Investigation of Therapeutic Potential of Isoform-Specific HDAC8 Inhibitors for the Treatment of Cutaneous T Cell Lymphoma.Anticancer Agents Med Chem. 2019;19(7):916-934. doi: 10.2174/1871520619666190301150254. Anticancer Agents Med Chem. 2019. PMID: 30836926
-
Novel Hydroxamic Acids Incorporating 1-((1H-1,2,3-Triazol-4-yl)methyl)- 3-substituted-2-oxoindolines: Synthesis, Biological Evaluation and SAR Analysis.Med Chem. 2018;14(8):831-850. doi: 10.2174/1573406414666180528111749. Med Chem. 2018. PMID: 29807520
-
Design, synthesis, and biological evaluation of indole-based hydroxamic acid derivatives as histone deacetylase inhibitors.Eur J Med Chem. 2022 Jan 5;227:113893. doi: 10.1016/j.ejmech.2021.113893. Epub 2021 Oct 2. Eur J Med Chem. 2022. PMID: 34656899
-
Histone deacetylase 8 (HDAC8) and its inhibitors with selectivity to other isoforms: An overview.Eur J Med Chem. 2019 Feb 15;164:214-240. doi: 10.1016/j.ejmech.2018.12.039. Epub 2018 Dec 19. Eur J Med Chem. 2019. PMID: 30594678 Review.
-
Histone deacetylase as a new target for cancer chemotherapy.Cancer Chemother Pharmacol. 2001 Aug;48 Suppl 1:S20-6. doi: 10.1007/s002800100300. Cancer Chemother Pharmacol. 2001. PMID: 11587361 Review.
Cited by
-
Design, synthesis and antifungal activity of novel matrine-hydroxamic acid derivatives containing benzene sulfonamide.RSC Adv. 2025 May 19;15(21):16510-16524. doi: 10.1039/d5ra01689d. eCollection 2025 May 15. RSC Adv. 2025. PMID: 40391361 Free PMC article.
References
LinkOut - more resources
Full Text Sources
