Double-blind randomized proof-of-concept trial of canakinumab in patients with COVID-19 associated cardiac injury and heightened inflammation
- PMID: 35923169
- PMCID: PMC9242054
- DOI: 10.1093/ehjopen/oeab002
Double-blind randomized proof-of-concept trial of canakinumab in patients with COVID-19 associated cardiac injury and heightened inflammation
Erratum in
-
Corrigendum to articles in EHJ Open missing data availability statements.Eur Heart J Open. 2024 Jan 16;4(1):oead137. doi: 10.1093/ehjopen/oead137. eCollection 2024 Jan. Eur Heart J Open. 2024. PMID: 38230360 Free PMC article.
Abstract
Aims: In coronavirus disease 2019 (COVID-19), myocardial injury is associated with systemic inflammation and higher mortality. Our aim was to perform a proof of concept trial with canakinumab, a monoclonal antibody to interleukin-1β, in patients with COVID-19, myocardial injury, and heightened inflammation.
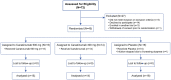
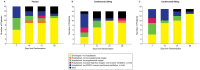
Methods and results: This trial required hospitalization due to COVID-19, elevated troponin, and a C-reactive protein concentration more than 50 mg/L. The primary endpoint was time to clinical improvement at Day 14, defined as either an improvement of two points on a seven-category ordinal scale or discharge from the hospital. The secondary endpoint was mortality at Day 28. Forty-five patients were randomly assigned to canakinumab 600 mg (n = 15), canakinumab 300 mg (n = 14), or placebo (n = 16). There was no difference in time to clinical improvement compared to placebo [recovery rate ratio (RRR) for canakinumab 600 mg 1.15, 95% confidence interval (CI) 0.46-2.91; RRR for canakinumab 300 mg 0.61, 95% CI 0.23-1.64]. At Day 28, 3 (18.8%) of 15 patients had died in the placebo group, compared with 3 (21.4%) of 14 patients with 300 mg canakinumab, and 1 (6.7%) of 15 patients with 600 mg canakinumab. There were no treatment-related deaths, and adverse events were similar between groups.
Conclusion: There was no difference in time to clinical improvement at Day 14 in patients treated with canakinumab, and no safety concerns were identified. Future studies could focus on high dose canakinumab in the treatment arm and assess efficacy outcomes at Day 28.
Keywords: COVID-19; Canakinumab; Interleukin-1; Myocardial injury.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2021. For permissions, please email: journals.permissions@oup.com.
Figures
Comment on
-
Targeting cytokine storm in COVID-19: what have we learned?Eur Heart J Open. 2021 Jul 29;1(1):oeab005. doi: 10.1093/ehjopen/oeab005. eCollection 2021 Aug. Eur Heart J Open. 2021. PMID: 35919093 Free PMC article. No abstract available.
References
-
- Biegel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19: preliminary report. N Engl J Med 2020;383:1813–1826. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials