A Turn-On Fluorescent Chemosensor for Cyanide Ion Detection in Real Water Samples
- PMID: 35923259
- PMCID: PMC9339681
- DOI: 10.3389/fchem.2022.923149
A Turn-On Fluorescent Chemosensor for Cyanide Ion Detection in Real Water Samples
Abstract
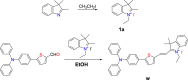
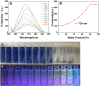
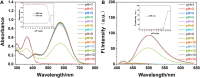
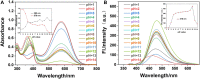
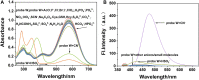
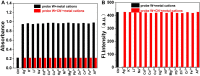
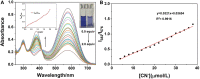
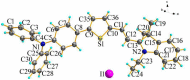
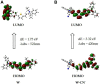
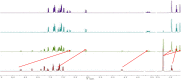
We have designed and synthesized a novel simple colorimetric fluorescent probe with aggregation-induced emission (AIE) properties. Probe 5-(4-(diphenylamine)phenyl) thiophen-2-formaldehyde W exhibited a turn-on fluorescent response to cyanide ion (CN-), which induces distinct visual color changes. Probe W exhibited a highly selective and sensitive ratiometric fluorescence response for the detection of CN- over a wide pH range (4-11) and in the presence of common interferents. The linear detection of CN- over the concentration range of 4.00-38.00 µM (R 2 = 0.9916, RSD = 0.02) was monitored by UV-Vis absorption spectrometry (UV-Vis) with the limit of detection determined to be 0.48 µM. The linear detection of CN- over the concentration range of 8.00-38.00 µM was examined by fluorescence spectrophotometry (R 2 = 0.99086, RSD = 0.031), and the detection limit was found to be 68.00 nM. The sensing mechanisms were confirmed by 1H NMR spectroscopic titrations, X-ray crystallographic analysis, and HRMS. Importantly, probe W was found to show rapid response, high selectivity, and sensitivity for cyanide anions in real water samples, over the range of 100.17∼100.86% in artificial lake water and 100.54∼101.64% in running water by UV-Vis absorption spectrometry, and over the range of 99.42∼100.71% in artificial lake water and 100.59∼101.17% in running water by fluorescence spectrophotometry. Importantly, this work provides a simple and effective approach which uses an economically cheap and uncomplicated synthetic route for the selective, sensitive, and quantitative detection of CN- ions in systems relevant to the environment and health.
Keywords: crystal structure; cyanide ion; fluorescent probe; real sample detection; synthesis.
Copyright © 2022 Shi, Wu, Shen, Zhou, Xu, Wang, Yang, Huang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
A Coumarin-Hemicyanine Deep Red Dye with a Large Stokes Shift for the Fluorescence Detection and Naked-Eye Recognition of Cyanide.Molecules. 2024 Jan 27;29(3):618. doi: 10.3390/molecules29030618. Molecules. 2024. PMID: 38338363 Free PMC article.
-
A reaction-based carbazole-dicyanovinyl conjugated colorimetric and ratiometric fluorescent probe for selective detection of cyanide ions.Spectrochim Acta A Mol Biomol Spectrosc. 2024 Jan 5;304:123350. doi: 10.1016/j.saa.2023.123350. Epub 2023 Sep 7. Spectrochim Acta A Mol Biomol Spectrosc. 2024. PMID: 37688886
-
Aggregation-Induced Emission-Based Material for Selective and Sensitive Recognition of Cyanide Anions in Solution and Biological Assays.ACS Omega. 2021 Jun 24;6(26):16704-16713. doi: 10.1021/acsomega.0c06080. eCollection 2021 Jul 6. ACS Omega. 2021. PMID: 34250330 Free PMC article.
-
A highly sensitive and selective fluorescent "off-on-off" relay chemosensor based on a new bis(salamo)-type tetraoxime for detecting Zn2+ and CN.Spectrochim Acta A Mol Biomol Spectrosc. 2019 Nov 5;222:117209. doi: 10.1016/j.saa.2019.117209. Epub 2019 Jun 3. Spectrochim Acta A Mol Biomol Spectrosc. 2019. PMID: 31200268 Review.
-
Molecular chemosensors for hazardous anions (AcO-, CN- and F-): progress in fluorescent and colorimetric detection strategies.Spectrochim Acta A Mol Biomol Spectrosc. 2025 Nov 15;341:126414. doi: 10.1016/j.saa.2025.126414. Epub 2025 May 16. Spectrochim Acta A Mol Biomol Spectrosc. 2025. PMID: 40398379 Review.
Cited by
-
Sensitive Optical Detection of Cyanide and Al(III) Ions and Development of a Simple Colourimetric Method for Estimation of Cyanide in Cassava Flour.J Fluoresc. 2025 May 5. doi: 10.1007/s10895-025-04348-1. Online ahead of print. J Fluoresc. 2025. PMID: 40323476
-
2-(N-Hexylcarbazole-3'-yl)-4-pyridinealdehyde: Cyanide Detection via Benzoin Condensation.Luminescence. 2025 Aug;40(8):e70274. doi: 10.1002/bio.70274. Luminescence. 2025. PMID: 40751326 Free PMC article.
-
A Simple Fluorescent Chemo-Dosimeter for Sensitive Detection of Cyanide and Hydrogen Sulfide Ions: Spectroscopic and TD-DFT Studies.J Fluoresc. 2025 Apr 23. doi: 10.1007/s10895-025-04295-x. Online ahead of print. J Fluoresc. 2025. PMID: 40266483
-
A Coumarin-Hemicyanine Deep Red Dye with a Large Stokes Shift for the Fluorescence Detection and Naked-Eye Recognition of Cyanide.Molecules. 2024 Jan 27;29(3):618. doi: 10.3390/molecules29030618. Molecules. 2024. PMID: 38338363 Free PMC article.
-
A coumarin-nicotinic hydrazone probe for chromofluorogenic detection of toxic cyanide ions and its application in molecular logic gate and real water samples analysis.Photochem Photobiol Sci. 2025 Apr;24(4):543-554. doi: 10.1007/s43630-025-00704-z. Epub 2025 Apr 1. Photochem Photobiol Sci. 2025. PMID: 40167971
References
-
- Afshani J., Badiei A., Jafari M., Shayesteh A., Karimi M., Lashgari N., et al. (2016). A Single Optical Sensor with High Sensitivity for Detection of Fe3+ and CN− Ions. J. Luminescence 179, 463–468. 10.1016/j.jlumin.2016.07.038 - DOI
-
- Chao J., Li Z., Zhang Y., Huo F., Yin C., Tong H., et al. (2016). A Ratiometric Fluorescence Probe for Monitoring Cyanide Ion in Live Cells. Sensors Actuators B Chem. 228, 192–199. 10.1016/j.snb.2016.01.033 - DOI
LinkOut - more resources
Full Text Sources

