Synthesis and Biological Evaluation of 3-(Pyridine-3-yl)-2-Oxazolidinone Derivatives as Antibacterial Agents
- PMID: 35923260
- PMCID: PMC9339906
- DOI: 10.3389/fchem.2022.949813
Synthesis and Biological Evaluation of 3-(Pyridine-3-yl)-2-Oxazolidinone Derivatives as Antibacterial Agents
Abstract
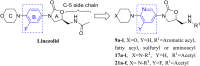
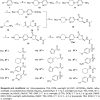
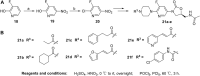
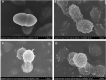
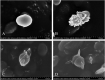
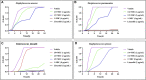
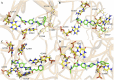
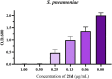
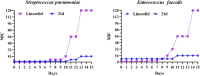
In this research, a series of 3-(pyridine-3-yl)-2-oxazolidinone derivatives was designed, synthesized, and evaluated for in vitro antibacterial activity, which included bacteriostatic, morphological, kinetic studies, and molecular docking. The results demonstrated that compounds 21b, 21d, 21e and 21f exhibited strong antibacterial activity similar to that of linezolid toward five Gram-positive bacteria. After observing the effect of the drug on the morphology and growth dynamics of the bacteria, the possible modes of action were predicted by molecular docking. Furthermore, the antibiofilm activity and the potential drug resistance assay was proceeded. These compounds exhibited universal antibiofilm activity and compound 21d showed significant concentration-dependent inhibition of biofilm formation. Compound 21d also showed a stable effect on S. pneumoniae (ATCC 49619) with less drug resistance growth for 15 days, which is much longer than that of linezolid. Overall, these results can be used to guide further exploration of novel antimicrobial agents.
Keywords: 3-(pyridine-3-yl)-2-oxazolidinone; antibacterial activity; biofilm formation inhibitory activity; drug resistance development; molecular docking.
Copyright © 2022 Jin, Wang, Chen, Liu, Zhang, Zhang, Sheng and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Optimization and Antibacterial Evaluation of Novel 3-(5-Fluoropyridine-3-yl)-2-oxazolidinone Derivatives Containing a Pyrimidine Substituted Piperazine.Molecules. 2023 May 23;28(11):4267. doi: 10.3390/molecules28114267. Molecules. 2023. PMID: 37298744 Free PMC article.
-
Synthesis and biological evaluation of novel 5-(hydroxamic acid)methyl oxazolidinone derivatives.Eur J Med Chem. 2015 Dec 1;106:120-31. doi: 10.1016/j.ejmech.2015.10.025. Epub 2015 Oct 23. Eur J Med Chem. 2015. PMID: 26536532
-
Oxazolidinone antimicrobials: a patent review (2012-2015).Expert Opin Ther Pat. 2016 May;26(5):591-605. doi: 10.1517/13543776.2016.1168807. Epub 2016 Apr 4. Expert Opin Ther Pat. 2016. PMID: 26998627 Review.
-
Genetic Determinants of Salmonella Resistance to the Biofilm-Inhibitory Effects of a Synthetic 4-Oxazolidinone Analog.Appl Environ Microbiol. 2020 Oct 1;86(20):e01120-20. doi: 10.1128/AEM.01120-20. Print 2020 Oct 1. Appl Environ Microbiol. 2020. PMID: 32769186 Free PMC article.
-
Tedizolid: a novel oxazolidinone with potent activity against multidrug-resistant gram-positive pathogens.Drugs. 2015 Feb;75(3):253-70. doi: 10.1007/s40265-015-0352-7. Drugs. 2015. PMID: 25673021 Review.
Cited by
-
Comparative Assessment of Docking Programs for Docking and Virtual Screening of Ribosomal Oxazolidinone Antibacterial Agents.Antibiotics (Basel). 2023 Feb 24;12(3):463. doi: 10.3390/antibiotics12030463. Antibiotics (Basel). 2023. PMID: 36978331 Free PMC article.
-
Optimization and Antibacterial Evaluation of Novel 3-(5-Fluoropyridine-3-yl)-2-oxazolidinone Derivatives Containing a Pyrimidine Substituted Piperazine.Molecules. 2023 May 23;28(11):4267. doi: 10.3390/molecules28114267. Molecules. 2023. PMID: 37298744 Free PMC article.
-
Recent Advances in Pyridine Scaffold: Focus on Chemistry, Synthesis, and Antibacterial Activities.Biomed Res Int. 2023 May 18;2023:9967591. doi: 10.1155/2023/9967591. eCollection 2023. Biomed Res Int. 2023. PMID: 37250749 Free PMC article. Review.
References
-
- Anbarasi K., Esther Mary S., Vijayakumar R., Krishnan P. (2020). Resistance Profile and Minimum Inhibitory Concentration versus Minimum Biofilm Inhibitory Concentration of Biofilm Positive Staphylococci. Int. J. Infect. Dis. 101, 30. 10.1016/j.ijid.2020.09.113 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

