Intestinal Flora-Derived Kynurenic Acid Protects Against Intestinal Damage Caused by Candida albicans Infection via Activation of Aryl Hydrocarbon Receptor
- PMID: 35923391
- PMCID: PMC9339982
- DOI: 10.3389/fmicb.2022.934786
Intestinal Flora-Derived Kynurenic Acid Protects Against Intestinal Damage Caused by Candida albicans Infection via Activation of Aryl Hydrocarbon Receptor
Abstract
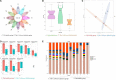
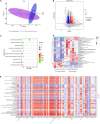
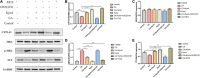
Colonization of the intestinal tract by Candida albicans (C. albicans) can lead to invasive candidiasis. Therefore, a functional intestinal epithelial barrier is critical for protecting against invasive C. albicans infections. We collected fecal samples from patients with Candida albicans bloodstream infection and healthy people. Through intestinal flora 16sRNA sequencing and intestinal metabolomic analysis, we found that C. albicans infection resulted in a significant decrease in the expression of the metabolite kynurenic acid (KynA). We used a repeated C. albicans intestinal infection mouse model, established following intake of 3% dextran sulfate sodium salt (DSS) for 9 days, and found that KynA, a tryptophan metabolite, inhibited inflammation, promoted expression of intestinal tight junction proteins, and protected from intestinal barrier damage caused by invasive Candida infections. We also demonstrated that KynA activated aryl hydrocarbon receptor (AHR) repressor in vivo and in vitro. Using Caco-2 cells co-cultured with C. albicans, we showed that KynA activated AHR, inhibited the myosin light chain kinase-phospho-myosin light chain (MLCK-pMLC) signaling pathway, and promoted tristetraprolin (TTP) expression to alleviate intestinal inflammation. Our findings suggest that the metabolite KynA which is differently expressed in patients with C. albicans infection and has a protective effect on the intestinal epithelium, via activating AHR, could be explored to provide new potential therapeutic strategies for invasive C. albicans infections.
Keywords: aryl hydrocarbon receptor; intestinal barrier function; intestinal flora; invasive C. albicans infections; kynurenic acid.
Copyright © 2022 Wang, Yin, Qi, Zhang, Zhu and Tang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Dinan T. G., Cryan J. F. (2017). The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. North Am. 46 77–89. - PubMed
LinkOut - more resources
Full Text Sources

