Immune Thrombocytopenia in Previously Healthy Individuals Following SARS-CoV-2 Vaccination (COVID-19 Immunization): A Descriptive Research of 70 Instances With a Focus on Biomarkers, Predictive Outcomes, and Consequences
- PMID: 35923492
- PMCID: PMC9342832
- DOI: 10.7759/cureus.26480
Immune Thrombocytopenia in Previously Healthy Individuals Following SARS-CoV-2 Vaccination (COVID-19 Immunization): A Descriptive Research of 70 Instances With a Focus on Biomarkers, Predictive Outcomes, and Consequences
Abstract
The coronavirus disease-2019 (COVID-19) pandemic is exacerbating the worldwide healthcare crisis. The pandemic has had an impact on nearly every system of our body. The Food and Drug Administration (FDA) gave immediate authorization of several vaccines to avoid critical COVID-19 outcomes following the rapid spread of the COVID-19. There have only been a few cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination-induced immune thrombocytopenia (ITP) so far. There should be enough information to identify whether some vaccination adverse effects, such as ITP, are caused by the vaccine. This study aims to determine how common ITP occurs after receiving the SARS-CoV-2 vaccine, as well as gender, age, symptoms, biomarkers, predicted outcomes, and sequelae. We looked at a number of research and compiled the best evidence of SARS-CoV-2 vaccine-induced thrombocytopenia currently available. To find the recommended reporting items, the search technique included keywords like "Immune thrombocytopenia," "COVID-19," "SARS-CoV-2," and "Vaccination." The search results were grouped using Boolean operators ("OR," "AND").
Keywords: covid-19; immune thrombocytopenia (itp); sars-cov-2; thrombosis; vaccination.
Copyright © 2022, Sharma et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


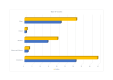
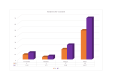


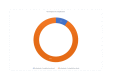
Similar articles
-
[Severe thrombocytopenia after COVID-19 mRNA vaccination].Rinsho Ketsueki. 2021;62(12):1684-1687. doi: 10.11406/rinketsu.62.1684. Rinsho Ketsueki. 2021. PMID: 35022337 Japanese.
-
Analysis of hematologic adverse events reported to a national surveillance system following COVID-19 bivalent booster vaccination.Ann Hematol. 2023 Apr;102(4):955-959. doi: 10.1007/s00277-023-05136-2. Epub 2023 Feb 16. Ann Hematol. 2023. PMID: 36795118 Free PMC article.
-
Autoimmune haemolytic anaemia and immune thrombocytopenia following SARS-CoV-2 and non-SARS-CoV-2 vaccination: 32 Years of passive surveillance data.Br J Haematol. 2023 Apr;201(2):227-233. doi: 10.1111/bjh.18627. Epub 2022 Dec 23. Br J Haematol. 2023. PMID: 36564040 Free PMC article.
-
A narrative review of anti-SARS-CoV-2 vaccines and immune thrombocytopenia: be aware, but reassured.Clin Adv Hematol Oncol. 2022 Sep;20(9):572-578. Clin Adv Hematol Oncol. 2022. PMID: 36125949 Review.
-
COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines.Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
Cited by
-
Autoimmune Diseases Affecting Hemostasis: A Narrative Review.Int J Mol Sci. 2022 Nov 25;23(23):14715. doi: 10.3390/ijms232314715. Int J Mol Sci. 2022. PMID: 36499042 Free PMC article. Review.
-
Cardiovascular Sequelae of the COVID-19 Vaccines.Cureus. 2025 Apr 10;17(4):e82041. doi: 10.7759/cureus.82041. eCollection 2025 Apr. Cureus. 2025. PMID: 40351947 Free PMC article. Review.
References
-
- WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ 2022
-
- Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Rodeghiero F, Stasi R, Gernsheimer T, et al. Blood. 2009;113:2386–2393. - PubMed
-
- Communicating the Potential Benefits and Harms of the Astra-Zeneca COVID-19 Vaccine. https://assets.publishing.service.gov.uk/government/uploads/system/uploa... 2022
-
- Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH. Oldenburg J, Klamroth R, Langer F, et al. Hamostaseologie. 2021;41:184–189. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
