Quantifying the Value of Introducing an Oral Drug Delivery Option for Edaravone: A Review of Analyses Evaluating the Economic Impact of Oral versus Intravenous Formulations
- PMID: 35923520
- PMCID: PMC9342658
- DOI: 10.2147/CEOR.S359025
Quantifying the Value of Introducing an Oral Drug Delivery Option for Edaravone: A Review of Analyses Evaluating the Economic Impact of Oral versus Intravenous Formulations
Abstract
Background: Drug formulation and route of administration can have an impact on not only patients' quality of life and disease outcomes but also costs of care. It is essential for decision makers to use appropriate economic modeling methods to guide drug coverage policies and to support patients' decision-making.
Purpose: To illustrate key cost considerations for decision makers in economic evaluation of innovative oral formulations as alternatives to intravenous medication.
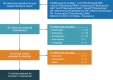
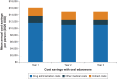
Materials and methods: A structured literature review was conducted using the PubMed database to examine methods used for quantifying the economic impact of introducing a new oral pharmaceutical formulation as an alternative to intravenous medication. To illustrate the methods described in this review, a cost-minimization analysis was conducted to quantify the impact of introducing an oral formulation of a medication originally developed as an intravenous treatment for amyotrophic lateral sclerosis.
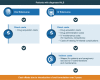
Results: We identified 14 published evaluations of oral and intravenous formulations from 10 countries across a variety of disease areas. The identified studies used cost-effectiveness (n=10), cost-minimization (n=2), and cost-calculation (n=2) modeling approaches. All but one (13/14) reported outcomes from payers' perspective, while societal perspectives were also incorporated in 3 of the reviewed evaluations. One study estimated costs from a public hospital's perspective. Only a subset of the identified studies accounted for the effects of safety (n=6) or efficacy (n=8) differences on treatment costs when estimating the costs of a formulation choice. Many studies that omitted these aspects did not include rationales for their decisions.
Conclusion: We found significant design variations in published models that estimated the impact of an additional formulation option on the treatment costs to payers and the society. Models need to be accompanied with clear descriptions on rationales for their time horizons and assumptions on how different formulations may affect healthcare costs from the selected perspectives.
Keywords: amyotrophic lateral sclerosis; cost-effectiveness analysis; cost-minimization; decision makers; formulation comparison.
© 2022 Ronquest et al.
Conflict of interest statement
Melissa Hagan and Malgorzata Ciepielewska have received personal compensation for serving as full-time employees of Mitsubishi Tanabe Pharma America, Inc. Naoko Ronquest, Kyle Paret, and Aaron Lucas are employees of RTI Health Solutions, which received consultancy fees from Mitsubishi Tanabe Pharma America, Inc. to conduct this study. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
[Cost-effectiveness analysis of schizophrenic patient care settings: impact of an atypical antipsychotic under long-acting injection formulation].Encephale. 2005 Mar-Apr;31(2):235-46. doi: 10.1016/s0013-7006(05)82390-5. Encephale. 2005. PMID: 15959450 Review. French.
-
Multi-gene Pharmacogenomic Testing That Includes Decision-Support Tools to Guide Medication Selection for Major Depression: A Health Technology Assessment.Ont Health Technol Assess Ser. 2021 Aug 12;21(13):1-214. eCollection 2021. Ont Health Technol Assess Ser. 2021. PMID: 34484487 Free PMC article.
-
Generalisability in economic evaluation studies in healthcare: a review and case studies.Health Technol Assess. 2004 Dec;8(49):iii-iv, 1-192. doi: 10.3310/hta8490. Health Technol Assess. 2004. PMID: 15544708 Review.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Intravenous iron for the treatment of iron deficiency anemia in China: a patient-level simulation model and cost-utility analysis comparing ferric derisomaltose with iron sucrose.J Med Econ. 2022 Jan-Dec;25(1):561-570. doi: 10.1080/13696998.2022.2065092. J Med Econ. 2022. PMID: 35403540
Cited by
-
Cost-utility analysis for sublingual versus intravenous edaravone in the treatment of amyotrophic lateral sclerosis.Orphanet J Rare Dis. 2024 Oct 28;19(1):400. doi: 10.1186/s13023-024-03381-w. Orphanet J Rare Dis. 2024. PMID: 39468607 Free PMC article.
-
Cost-effectiveness of iptacopan for paroxysmal nocturnal hemoglobinuria.Blood. 2025 Jan 2;145(1):127-140. doi: 10.1182/blood.2024025176. Blood. 2025. PMID: 39374533 Free PMC article.
References
-
- Institute for Clinical and Economic Review. 2020–2023 value assessment framework; 2020. Available from: https://icer.org/wp-content/uploads/2021/03/ICER_2020_2023_VAF_013120-4-.... Accessed June 7, 2021.
-
- Seidman J, Gaur P, Boler C The patient-perspective value framework: short- and long-term recommendations to influence value assessment methodology; 2019. Available from: https://avalere.com/wp-content/uploads/2019/05/20190530_PPVF-Working-Gro.... Accessed June 7, 2021.
Publication types
LinkOut - more resources
Full Text Sources