Tauopathy and Epilepsy Comorbidities and Underlying Mechanisms
- PMID: 35923547
- PMCID: PMC9340804
- DOI: 10.3389/fnagi.2022.903973
Tauopathy and Epilepsy Comorbidities and Underlying Mechanisms
Abstract
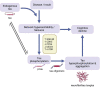
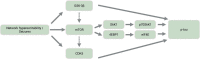
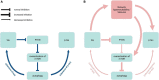
Tau is a microtubule-associated protein known to bind and promote assembly of microtubules in neurons under physiological conditions. However, under pathological conditions, aggregation of hyperphosphorylated tau causes neuronal toxicity, neurodegeneration, and resulting tauopathies like Alzheimer's disease (AD). Clinically, patients with tauopathies present with either dementia, movement disorders, or a combination of both. The deposition of hyperphosphorylated tau in the brain is also associated with epilepsy and network hyperexcitability in a variety of neurological diseases. Furthermore, pharmacological and genetic targeting of tau-based mechanisms can have anti-seizure effects. Suppressing tau phosphorylation decreases seizure activity in acquired epilepsy models while reducing or ablating tau attenuates network hyperexcitability in both Alzheimer's and epilepsy models. However, it remains unclear whether tauopathy and epilepsy comorbidities are mediated by convergent mechanisms occurring upstream of epileptogenesis and tau aggregation, by feedforward mechanisms between the two, or simply by coincident processes. In this review, we investigate the relationship between tauopathies and seizure disorders, including temporal lobe epilepsy (TLE), post-traumatic epilepsy (PTE), autism spectrum disorder (ASD), Dravet syndrome, Nodding syndrome, Niemann-Pick type C disease (NPC), Lafora disease, focal cortical dysplasia, and tuberous sclerosis complex. We also explore potential mechanisms implicating the role of tau kinases and phosphatases as well as the mammalian target of rapamycin (mTOR) in the promotion of co-pathology. Understanding the role of these co-pathologies could lead to new insights and therapies targeting both epileptogenic mechanisms and cognitive decline.
Keywords: cognitive decline; epilepsy; hyperexcitability; hyperphosphorylation of tau; mTOR; tau.
Copyright © 2022 Hwang, Vaknalli, Addo-Osafo, Vicente and Vossel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

