Evaluation of Access Disparities to Biologic Disease-Modifying Antirheumatic Drugs in Rural and Urban Communities
- PMID: 35923666
- PMCID: PMC9339343
- DOI: 10.7759/cureus.26448
Evaluation of Access Disparities to Biologic Disease-Modifying Antirheumatic Drugs in Rural and Urban Communities
Abstract
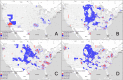
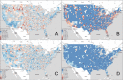
The American College of Rheumatology guidelines provides a strong recommendation for the use of biologic disease-modifying antirheumatic drugs (bDMARDs) when conventional rheumatoid arthritis treatments fail to meet treatment targets. Although bDMARDs are an effective and important treatment component, access inequalities remain a challenge in many communities worldwide. The purpose of this analysis is to assess nationwide trends in bDMARD access in the United States, with a specific focus on rural and urban access gaps. This study combined multiple county-level databases to assess bDMARD prescriptions from 2015 to 2019. Using geospatial analysis and the Moran's I statistic, counties were classified according to prescription levels to assess for hotspots and coldspots. Analysis of variance (ANOVA) was used to compare significant counties across 49 socioeconomic variables of interest. The analysis identified statistically significant hotspot and coldspot prescription clusters within the United States. Coldspot (Low-Low) clusters with low access to bDMARDs are located predominantly in the rural west North Central region, extending down to Oklahoma and Arkansas. Hotspot (High-High) clusters are seen in urban and metro areas of Wisconsin, Minnesota, Pennsylvania, North Carolina, Georgia, Oregon, and the southern tip of Texas. Comparing coldspot to hotspot areas of bDMARD access revealed that the Medicare populations were older, more rural, less educated, less impoverished, and less likely to get their bDMARDs from a rheumatologist.
Keywords: disease-modifying antirheumatic drugs; geospatial analysis; healthcare inequality; medicare data; rural vs metropolitan.
Copyright © 2022, Peterman et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Singh JA, Saag KG, Bridges SL Jr, et al. Arthritis Rheumatol. 2016;68:1–26. - PubMed
-
- Strategies toward rheumatoid arthritis therapy; the old and the new. Abbasi M, Mousavi MJ, Jamalzehi S, Alimohammadi R, Bezvan MH, Mohammadi H, Aslani S. J Cell Physiol. 2019;234:10018–10031. - PubMed
-
- Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Maini R, St Clair EW, Breedveld F, et al. Lancet Lond Engl. 1999;354:1932–1939. - PubMed
LinkOut - more resources
Full Text Sources
