Development of subtype-selective covalent ligands for the adenosine A2B receptor by tuning the reactive group
- PMID: 35923720
- PMCID: PMC9298184
- DOI: 10.1039/d2md00132b
Development of subtype-selective covalent ligands for the adenosine A2B receptor by tuning the reactive group
Abstract
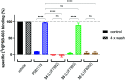
Signalling through the adenosine receptors (ARs), in particular through the adenosine A2B receptor (A2BAR), has been shown to play a role in a variety of pathological conditions, ranging from immune disorders to cancer. Covalent ligands for the A2BAR have the potential to irreversibly block the receptor, as well as inhibit all A2BAR-induced signalling pathways. This will allow a thorough investigation of the pathophysiological role of the receptor. In this study, we synthesized and evaluated a set of potential covalent ligands for the A2BAR. The ligands all contain a core scaffold consisting of a substituted xanthine, varying in type and orientation of electrophilic group (warhead). Here, we find that the right combination of these variables is necessary for a high affinity, irreversible mode of binding and selectivity towards the A2BAR. Altogether, this is the case for sulfonyl fluoride 24 (LUF7982), a covalent ligand that allows for novel ways to interrogate the A2BAR.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
N-Acyl-N-Alkyl Sulfonamide Probes for Ligand-Directed Covalent Labeling of GPCRs: The Adenosine A2B Receptor as Case Study.ACS Chem Biol. 2024 Jul 19;19(7):1554-1562. doi: 10.1021/acschembio.4c00210. Epub 2024 Jun 26. ACS Chem Biol. 2024. PMID: 38920052 Free PMC article.
-
Irreversible Antagonists for the Adenosine A2B Receptor.Molecules. 2022 Jun 13;27(12):3792. doi: 10.3390/molecules27123792. Molecules. 2022. PMID: 35744918 Free PMC article.
-
The hybrid molecule, VCP746, is a potent adenosine A2B receptor agonist that stimulates anti-fibrotic signalling.Biochem Pharmacol. 2016 Oct 1;117:46-56. doi: 10.1016/j.bcp.2016.08.007. Epub 2016 Aug 9. Biochem Pharmacol. 2016. PMID: 27520486
-
The Many Faces of the A2b Adenosine Receptor in Cardiovascular and Metabolic Diseases.J Cell Physiol. 2015 Dec;230(12):2891-7. doi: 10.1002/jcp.25043. J Cell Physiol. 2015. PMID: 25975415 Free PMC article. Review.
-
The adenosine A2B G protein-coupled receptor: Recent advances and therapeutic implications.Pharmacol Ther. 2019 Jun;198:20-33. doi: 10.1016/j.pharmthera.2019.01.003. Epub 2019 Jan 22. Pharmacol Ther. 2019. PMID: 30677476 Review.
Cited by
-
Preparation of 18F-Labeled Tracers Targeting Fibroblast Activation Protein via Sulfur [18F]Fluoride Exchange Reaction.Pharmaceutics. 2023 Dec 10;15(12):2749. doi: 10.3390/pharmaceutics15122749. Pharmaceutics. 2023. PMID: 38140090 Free PMC article.
-
Sulfur fluoride exchange.Nat Rev Methods Primers. 2023;3:58. Epub 2023 Aug 3. Nat Rev Methods Primers. 2023. PMID: 38873592 Free PMC article.
-
N-Acyl-N-Alkyl Sulfonamide Probes for Ligand-Directed Covalent Labeling of GPCRs: The Adenosine A2B Receptor as Case Study.ACS Chem Biol. 2024 Jul 19;19(7):1554-1562. doi: 10.1021/acschembio.4c00210. Epub 2024 Jun 26. ACS Chem Biol. 2024. PMID: 38920052 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Chemical Information
Research Materials

