Role of CT in the Pre- and Postoperative Assessment of Conotruncal Anomalies
- PMID: 35923747
- PMCID: PMC9308465
- DOI: 10.1148/ryct.210089
Role of CT in the Pre- and Postoperative Assessment of Conotruncal Anomalies
Abstract
Conotruncal anomalies, also referred to as outflow tract anomalies, are congenital heart defects that result from abnormal septation of the great vessels' outflow tracts. The major conotruncal anomalies include tetralogy of Fallot, double-outlet right ventricle, transposition of the great arteries, truncus arteriosus, and interrupted aortic arch. Other defects, which are often components of the major anomalies, include pulmonary atresia with ventricular septal defect, pulmonary valve agenesis, aortopulmonary window, and double-outlet left ventricle. CT has emerged as a robust diagnostic tool in preoperative and postoperative assessment of various congenital heart diseases, including conotruncal anomalies. The data provided with multidetector CT imaging are useful for treatment planning and follow-up monitoring after surgery or intervention. Unlike echocardiography and MRI, CT is not limited by a small acoustic window, metallic devices, and need for sedation or anesthesia. Major advances in CT equipment, including dual-source scanners, wide-detector scanners, high-efficiency detectors, higher x-ray tube power, automatic tube current modulation, and advanced three-dimensional postprocessing, provide a low-risk, high-quality alternative to diagnostic cardiac catheterization and MRI. This review explores the various conotruncal anomalies and elucidates the role of CT imaging in their pre- and postoperative assessment. Keywords: CT, CT Angiography, Stents, Pediatrics © RSNA, 2022.
Keywords: CT; CT Angiography; Pediatrics; Stents.
© 2022 by the Radiological Society of North America, Inc.
Conflict of interest statement
Disclosures of conflicts of interest: P.K. No relevant relationships. M.B. No relevant relationships.
Figures
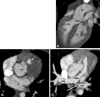
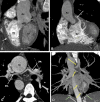
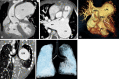
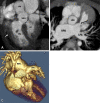

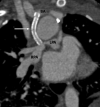
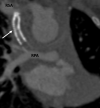
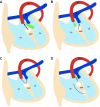
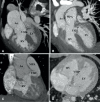
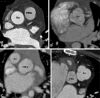
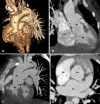
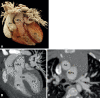
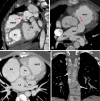
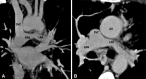
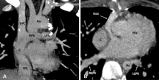
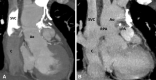
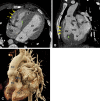
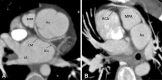
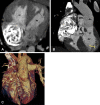
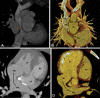

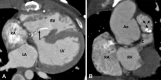
Similar articles
-
Cardiovascular MR imaging of conotruncal anomalies.Radiographics. 2010 Jul-Aug;30(4):1069-94. doi: 10.1148/rg.304095158. Radiographics. 2010. PMID: 20631369
-
Biventricular repair of conotruncal anomalies associated with aortic arch obstruction: 103 patients.Circulation. 1997 Nov 4;96(9 Suppl):II-328-34. Circulation. 1997. PMID: 9386119
-
Role of Computed Tomography in Pre- and Postoperative Evaluation of a Double-Outlet Right Ventricle.J Cardiovasc Imaging. 2021 Jul;29(3):205-227. doi: 10.4250/jcvi.2020.0196. Epub 2021 Mar 8. J Cardiovasc Imaging. 2021. PMID: 34080329 Free PMC article. Review.
-
Chromosome 22q11.2 microdeletion in children with conotruncal heart defects: frequency, associated cardiovascular anomalies, and outcome following cardiac surgery.Eur J Pediatr. 2008 Oct;167(10):1135-40. doi: 10.1007/s00431-007-0645-2. Epub 2008 Jan 3. Eur J Pediatr. 2008. PMID: 18172682
-
Magnetic resonance imaging evaluation of congenital heart disease: conotruncal anomalies.J Cardiovasc Magn Reson. 2006;8(4):645-59. doi: 10.1080/10976640600721544. J Cardiovasc Magn Reson. 2006. PMID: 16869316 Review.
Cited by
-
Computed Tomographic Evaluation of Congenital Left Ventricular Outflow Obstruction.Curr Cardiol Rev. 2023;19(6):31-49. doi: 10.2174/1573403X19666230525144602. Curr Cardiol Rev. 2023. PMID: 37231752 Free PMC article.
-
Multimodality Imaging Assessment of Tetralogy of Fallot: From Diagnosis to Long-Term Follow-Up.Children (Basel). 2023 Oct 27;10(11):1747. doi: 10.3390/children10111747. Children (Basel). 2023. PMID: 38002838 Free PMC article. Review.
-
Anatomical and Micro-CT measurement analysis of ocular volume and intraocular volume in adult Bama Miniature pigs, New Zealand rabbits, and Sprague-Dawley rats.PLoS One. 2024 Sep 20;19(9):e0310830. doi: 10.1371/journal.pone.0310830. eCollection 2024. PLoS One. 2024. PMID: 39302918 Free PMC article.
-
Rare Sinusal Variant of Truncus Arteriosus with Discontinuous Pulmonary Arteries.Indian J Radiol Imaging. 2023 May 6;33(3):424-425. doi: 10.1055/s-0043-1767693. eCollection 2023 Jul. Indian J Radiol Imaging. 2023. PMID: 37362376 Free PMC article. No abstract available.
-
Impact of 3D Printing on Cardiac Surgery in Congenital Heart Diseases: A Systematic Review and Meta-Analysis.Arq Bras Cardiol. 2024 Nov;121(12):e20240430. doi: 10.36660/abc.20240430. Arq Bras Cardiol. 2024. PMID: 39968976 Free PMC article. English, Portuguese.
References
-
- Restivo A , Piacentini G , Placidi S , Saffirio C , Marino B . Cardiac outflow tract: a review of some embryogenetic aspects of the conotruncal region of the heart . Anat Rec A Discov Mol Cell Evol Biol 2006. ; 288 ( 9 ): 936 – 943 . - PubMed
-
- Mcleod G , Shum K , Gupta T , et al. . Echocardiography in congenital heart disease . Prog Cardiovasc Dis 2018. ; 61 ( 5-6 ): 468 – 475 . - PubMed
-
- Frank L , Dillman JR , Parish V , et al. . Cardiovascular MR imaging of conotruncal anomalies . RadioGraphics 2010. ; 30 ( 4 ): 1069 – 1094 . - PubMed
-
- Goo HW , Park IS , Ko JK , Kim YH , Seo DM , Park JJ . Computed tomography for the diagnosis of congenital heart disease in pediatric and adult patients . Int J Cardiovasc Imaging 2005. ; 21 ( 2-3 ): 347 – 365 ; discussion 367. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

