Evidence of Neutralizing and Non-Neutralizing Anti-Glucosaminidase Antibodies in Patients With S. Aureus Osteomyelitis and Their Association With Clinical Outcome Following Surgery in a Clinical Pilot
- PMID: 35923804
- PMCID: PMC9339635
- DOI: 10.3389/fcimb.2022.876898
Evidence of Neutralizing and Non-Neutralizing Anti-Glucosaminidase Antibodies in Patients With S. Aureus Osteomyelitis and Their Association With Clinical Outcome Following Surgery in a Clinical Pilot
Abstract
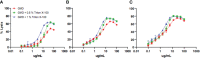
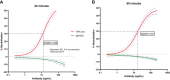
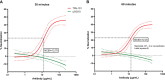
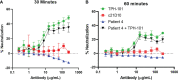
Staphylococcus aureus osteomyelitis remains a very challenging condition; recent clinical studies have shown infection control rates following surgery/antibiotics to be ~60%. Additionally, prior efforts to produce an effective S. aureus vaccine have failed, in part due to lack of knowledge of protective immunity. Previously, we demonstrated that anti-glucosaminidase (Gmd) antibodies are protective in animal models but found that only 6.7% of culture-confirmed S. aureus osteomyelitis patients in the AO Clinical Priority Program (AO-CPP) Registry had basal serum levels (>10 ng/ml) of anti-Gmd at the time of surgery (baseline). We identified a small subset of patients with high levels of anti-Gmd antibodies and adverse outcomes following surgery, not explained by Ig class switching to non-functional isotypes. Here, we aimed to test the hypothesis that clinical cure following surgery is associated with anti-Gmd neutralizing antibodies in serum. Therefore, we first optimized an in vitro assay that quantifies recombinant Gmd lysis of the M. luteus cell wall and used it to demonstrate the 50% neutralizing concentration (NC50) of a humanized anti-Gmd mAb (TPH-101) to be ~15.6 μg/ml. We also demonstrated that human serum deficient in anti-Gmd antibodies can be complemented by TPH-101 to achieve the same dose-dependent Gmd neutralizing activity as purified TPH-101. Finally, we assessed the anti-Gmd physical titer and neutralizing activity in sera from 11 patients in the AO-CPP Registry, who were characterized into four groups post-hoc. Group 1 patients (n=3) had high anti-Gmd physical and neutralizing titers at baseline that decreased with clinical cure of the infection over time. Group 2 patients (n=3) had undetectable anti-Gmd antibodies throughout the study and adverse outcomes. Group 3 (n=3) had high titers +/- neutralizing anti-Gmd at baseline with adverse outcomes. Group 4 (n=2) had low titers of non-neutralizing anti-Gmd at baseline with delayed high titers and adverse outcomes. Collectively, these findings demonstrate that both neutralizing and non-neutralizing anti-Gmd antibodies exist in S. aureus osteomyelitis patients and that screening for these antibodies could have a value for identifying patients in need of passive immunization prior to surgery. Future prospective studies to test the prognostic value of anti-Gmd antibodies to assess the potential of passive immunization with TPH-101 are warranted.
Keywords: Staphylococcus aureus; antibodies; glucosaminidase; immunoassay; osteomyelitis.
Copyright © 2022 Sherchand, Adhikari, Muthukrishnan, Kanipakala, Owen, Xie, Aman, Proctor, Schwarz and Kates.
Conflict of interest statement
SS, RA, TK, and MA are paid employees of Integrated Biotherapeutics Inc. RP and ES are paid consultants of Integrated Biotherapeutics Inc. and have stock in Telephus, LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Lack of Humoral Immunity Against Glucosaminidase Is Associated with Postoperative Complications in Staphylococcus aureus Osteomyelitis.J Bone Joint Surg Am. 2020 Nov 4;102(21):1842-1848. doi: 10.2106/JBJS.20.00029. J Bone Joint Surg Am. 2020. PMID: 32858560 Free PMC article.
-
Deriving a dose and regimen for anti-glucosaminidase antibody passive-immunisation for patients with Staphylococcus aureus osteomyelitis.Eur Cell Mater. 2020 Jan 31;39:96-107. doi: 10.22203/eCM.v039a06. Eur Cell Mater. 2020. PMID: 32003439 Free PMC article.
-
Anti-glucosaminidase IgG in sera as a biomarker of host immunity against Staphylococcus aureus in orthopaedic surgery patients.J Bone Joint Surg Am. 2013 Nov 20;95(22):e171. doi: 10.2106/JBJS.L.01654. J Bone Joint Surg Am. 2013. PMID: 24257671 Free PMC article.
-
The impact of methicillin resistance on clinical outcome among patients with Staphylococcus aureus osteomyelitis: a retrospective cohort study of 482 cases.Sci Rep. 2023 May 17;13(1):7990. doi: 10.1038/s41598-023-35111-w. Sci Rep. 2023. PMID: 37198265 Free PMC article. Review.
-
The effect of Staphylococcus aureus on innate and adaptive immunity and potential immunotherapy for S. aureus-induced osteomyelitis.Front Immunol. 2023 Sep 8;14:1219895. doi: 10.3389/fimmu.2023.1219895. eCollection 2023. Front Immunol. 2023. PMID: 37744377 Free PMC article. Review.
Cited by
-
Staphylococcus aureus adaptive evolution: Recent insights on how immune evasion, immunometabolic subversion and host genetics impact vaccine development.Front Cell Infect Microbiol. 2022 Dec 27;12:1060810. doi: 10.3389/fcimb.2022.1060810. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36636720 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

