Citicoline Treatment in Acute Ischemic Stroke: A Randomized, Single-Blind TMS Study
- PMID: 35923827
- PMCID: PMC9340348
- DOI: 10.3389/fneur.2022.915362
Citicoline Treatment in Acute Ischemic Stroke: A Randomized, Single-Blind TMS Study
Abstract
Background: Recent research on animal models of ischemic stroke supports the idea that pharmacological treatment potentially enhancing intrinsic brain plasticity could modulate acute brain damage, with improved functional recovery. One of these new drugs is citicoline, which could provide neurovascular protection and repair effects.
Objectives: The objective of this randomized, single-blind experimental study was to evaluate whether the treatment with Rischiaril® Forte was able to restore intracortical excitability measures, evaluated through transcranial magnetic stimulation (TMS) protocols, in patients with acute ischemic stroke.
Methods: Patients with acute ischemic stroke were recruited and assigned to an eight-week therapy of standard treatment (control group - CG) or CDP-choline (Rischiaril® Forte, containing 1,000 mg of citicoline sodium salt) added to conventional treatment (treatment group - TG). Each subject underwent a clinical evaluation and neurophysiological assessment using TMS, pretretament and posttreatment.
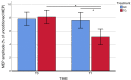
Results: A total of thirty participants (mean [SD] age, 68.1 [9.6] years; 11 women [37%]) completed the study. We did not observe significant changes in clinical scores after CDP-choline treatment (all p > 0.05), but we observed a significant improvement in short-interval intracortical inhibition (SAI) (p = 0.003) in the TG group compared to the CG group.
Conclusions: The eight-week treatment with citicoline after acute ischemic stroke may restore intracortical excitability measures, which partially depends on cholinergic transmission. This study extends current knowledge of the application of citicoline in acute ischemic stroke.
Keywords: cholinergic system (CS); citicoline; short-latency afferent inhibition (SAI); stroke; transcranial magnetic stimulation.
Copyright © 2022 Premi, Cantoni, Benussi, Gilberti, Vergani, Delrio, Gamba, Spezi, Costa, Padovani, Borroni and Magoni.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG, et al. . Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. (2021) 20:1–26. 10.1016/S1474-4422(21)00252-0 - DOI - PMC - PubMed
-
- Dávalos A, Alvarez-Sabín J, Castillo J, Díez-Tejedor E, Ferro J, Martínez-Vila E, et al. . Citicoline in the treatment of acute ischaemic stroke: an international, randomised, multicentre, placebo-controlled study (ICTUS trial). Lancet. (2012) 380:349–57. 10.1016/S0140-6736(12)60813-7 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous