Extracellular vimentin is expressed at the rear of activated macrophage-like cells: Potential role in enhancement of migration and phagocytosis
- PMID: 35923851
- PMCID: PMC9340215
- DOI: 10.3389/fcell.2022.891281
Extracellular vimentin is expressed at the rear of activated macrophage-like cells: Potential role in enhancement of migration and phagocytosis
Abstract
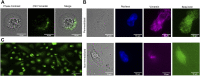
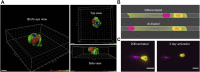
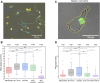
Macrophages have a vital role in the immune system through elimination of cell debris and microorganisms by phagocytosis. The activation of macrophages by tumour necrosis factor-α induces expression of extracellular cell-surface vimentin and promotes release of this vimentin into the extracellular environment. Vimentin is a cytoskeletal protein that is primarily located in the cytoplasm of cells. However, under circumstances like injury, stress, senescence and activation, vimentin can be expressed on the extracellular cell surface, or it can be released into the extracellular space. The characteristics of this extracellular vimentin, and its implications for the functional role of macrophages and the mechanism of secretion remain unclear. Here, we demonstrate that vimentin is released mainly from the back of macrophage-like cells. This polarisation is strongly enhanced upon macrophage activation. One-dimensional patterned lines showed that extracellular cell-surface vimentin is localised primarily at the back of activated macrophage-like cells. Through two-dimensional migration and phagocytosis assays, we show that this extracellular vimentin enhances migration and phagocytosis of macrophage-like cells. We further show that this extracellular vimentin forms agglomerates on the cell surface, in contrast to its intracellular filamentous form, and that it is released into the extracellular space in the form of small fragments. Taken together, we provide new insights into the release of extracellular cell-surface vimentin and its implications for macrophage functionality.
Keywords: activation; extracellular vimentin; macrophages; migration; phagocytosis; polarisation; vimentin secretion.
Copyright © 2022 Thalla, Rajwar, Laurent, Becher, Kainka and Lautenschläger.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Oxidized LDL induces vimentin secretion by macrophages and contributes to atherosclerotic inflammation.J Mol Med (Berl). 2020 Jul;98(7):973-983. doi: 10.1007/s00109-020-01923-w. Epub 2020 May 25. J Mol Med (Berl). 2020. PMID: 32451671
-
Role of Extracellular Vimentin in Cancer-Cell Functionality and Its Influence on Cell Monolayer Permeability Changes Induced by SARS-CoV-2 Receptor Binding Domain.Int J Mol Sci. 2021 Jul 12;22(14):7469. doi: 10.3390/ijms22147469. Int J Mol Sci. 2021. PMID: 34299089 Free PMC article.
-
Extracellular vimentin: Battle between the devil and the angel.Curr Opin Cell Biol. 2023 Dec;85:102265. doi: 10.1016/j.ceb.2023.102265. Epub 2023 Oct 29. Curr Opin Cell Biol. 2023. PMID: 37866018 Review.
-
Resident murine macrophage migration and phagocytosis are modulated by growth hormone.Cell Biol Int. 2018 May;42(5):615-623. doi: 10.1002/cbin.10939. Epub 2018 Feb 6. Cell Biol Int. 2018. PMID: 29363842
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
Cited by
-
Septin 9 expression regulates 'don't eat me' signals and identifies an immune-epithelial class of intrahepatic cholangiocarcinoma.Mol Oncol. 2024 Oct;18(10):2369-2392. doi: 10.1002/1878-0261.13673. Epub 2024 Jul 31. Mol Oncol. 2024. PMID: 39082897 Free PMC article.
-
Vimentin: from a cytoskeletal protein to a critical modulator of immune response and a target for infection.Front Immunol. 2023 Jul 5;14:1224352. doi: 10.3389/fimmu.2023.1224352. eCollection 2023. Front Immunol. 2023. PMID: 37475865 Free PMC article. Review.
-
AXL-initiated paracrine activation of pSTAT3 enhances mesenchymal and vasculogenic supportive features of tumor-associated macrophages.Cell Rep. 2023 Sep 26;42(9):113067. doi: 10.1016/j.celrep.2023.113067. Epub 2023 Sep 1. Cell Rep. 2023. PMID: 37659081 Free PMC article.
-
Annexin A2 Contributes to Release of Extracellular Vimentin in Response to Inflammation.FASEB J. 2025 May 15;39(9):e70621. doi: 10.1096/fj.202500793R. FASEB J. 2025. PMID: 40346842 Free PMC article.
-
Extracellular vimentin is a damage-associated molecular pattern protein serving as an agonist of TLR4 in human neutrophils.Cell Commun Signal. 2025 Feb 5;23(1):64. doi: 10.1186/s12964-025-02062-w. Cell Commun Signal. 2025. PMID: 39910535 Free PMC article.
References
-
- Collins H. L., Bancroft G. J. (1992). Cytokine enhancement of complement-dependent phagocytosis by macrophages: Synergy of tumor necrosis factor-α and granulocyte-macrophage colony-stimulating factor for phagocytosis of Cryptococcus neoformans. Eur. J. Immunol. 22, 1447–1454. 10.1002/eji.1830220617 - DOI - PubMed
LinkOut - more resources
Full Text Sources

