Perspective of Melatonin-Mediated Stress Resilience and Cu Remediation Efficiency of Brassica juncea in Cu-Contaminated Soils
- PMID: 35923886
- PMCID: PMC9340790
- DOI: 10.3389/fpls.2022.910714
Perspective of Melatonin-Mediated Stress Resilience and Cu Remediation Efficiency of Brassica juncea in Cu-Contaminated Soils
Abstract
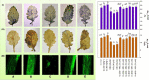
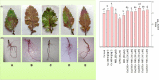
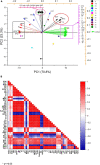
The present study evaluated the influence of melatonin (MEL) on copper toxicity in terms of morphophysiological, microscopic, histochemical, and stress resilience responses in Brassica juncea. Different levels of Cu (0, 30, and 60 mg kg-1) were given in air-dried soil, and 25 days after sowing (DAS), plants were sprayed with 30, 40, or 50 μM of MEL. The results demonstrated that under Cu stress, a significant amount of Cu accumulated in plant tissues, particularly in roots than in upper ground tissues, thereby suppressing the overall growth as evidenced by decrease in tolerance index and photosynthesis and increase in oxidative stress biomarkers (reactive oxygen species, malondialdehyde, and electrolyte leakage content) and cell death. Interestingly, the follow-up treatment of MEL, mainly 40 μM, efficiently improved the physio-biochemical and growth parameters, sugar accumulation, and metabolism. The potential of MEL in modulating Cu stress is attributed to its involvement in enriching the level of nutrient and improving chloroplast and stomatal organization besides lowering oxidative stress via enhanced levels of antioxidants. MEL improved the Cu reclamation potential in plants by enhancing Cu uptake and its translocation to aerial tissues. Principal component analysis showed that most of the morphophysiological and growth attributes were positively linked with MEL and negatively related to Cu levels, whereas all the stress-enhancing attributes showed a strong relationship with excessive Cu levels in soils. The present study suggested that MEL has the potential to improve growth and photosynthesis resulting in improved stress resilience under Cu stress along with increased remediation capability of mustard for remediation of Cu-contaminated soils.
Keywords: antioxidant; bioaccumulation; chloroplast; oxidative stress; phytoremediation.
Copyright © 2022 Mir, Alam and Hayat.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Auxin regulates growth, photosynthetic efficiency and mitigates copper induced toxicity via modulation of nutrient status, sugar metabolism and antioxidant potential in Brassica juncea.Plant Physiol Biochem. 2022 Aug 15;185:244-259. doi: 10.1016/j.plaphy.2022.06.006. Epub 2022 Jun 8. Plant Physiol Biochem. 2022. PMID: 35717733
-
Melatonin modulates photosynthesis, redox status, and elemental composition to promote growth of Brassica juncea-a dose-dependent effect.Protoplasma. 2020 Nov;257(6):1685-1700. doi: 10.1007/s00709-020-01537-6. Epub 2020 Aug 11. Protoplasma. 2020. PMID: 32778964
-
Synergistic impact of two autochthonous saprobic fungi (A. niger and T. pseudokoningii) on the growth, ionic contents, and metals uptake in Brassica juncea L. and Vigna radiata L. under tannery solid waste contaminated soil.Int J Phytoremediation. 2023;25(11):1488-1500. doi: 10.1080/15226514.2023.2166457. Epub 2023 Jan 12. Int J Phytoremediation. 2023. PMID: 36633455
-
Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress.J Hazard Mater. 2020 Nov 5;398:122882. doi: 10.1016/j.jhazmat.2020.122882. Epub 2020 May 15. J Hazard Mater. 2020. PMID: 32516727
-
Screening of various Brassica species for phytoremediation of heavy metals-contaminated soil of Lakki Marwat, Pakistan.Environ Sci Pollut Res Int. 2022 May;29(25):37765-37776. doi: 10.1007/s11356-021-18109-7. Epub 2022 Jan 24. Environ Sci Pollut Res Int. 2022. PMID: 35075562
Cited by
-
Systemic role of melatonin in enhancing temperature stress tolerance in fenugreek: coordination of antioxidant defense, hormonal regulation, energy status, sulfur metabolism, and diosgenin pathway genes.BMC Plant Biol. 2025 Aug 26;25(1):1131. doi: 10.1186/s12870-025-07224-z. BMC Plant Biol. 2025. PMID: 40859195 Free PMC article.
-
Genotype-dependent resilience mediated by melatonin in sweet corn.BMC Plant Biol. 2025 Jan 8;25(1):29. doi: 10.1186/s12870-024-05972-y. BMC Plant Biol. 2025. PMID: 39773623 Free PMC article.
-
Characterization of Two-Component System gene (TCS) in melatonin-treated common bean under salt and drought stress.Physiol Mol Biol Plants. 2023 Nov;29(11):1733-1754. doi: 10.1007/s12298-023-01406-5. Epub 2023 Dec 23. Physiol Mol Biol Plants. 2023. PMID: 38162914 Free PMC article.
-
Enhancing growth, vitality, and aromatic richness: unveiling the dual magic of silicon dioxide and titanium dioxide nanoparticles in Ocimum tenuiflorum L.Front Plant Sci. 2024 Feb 6;15:1335965. doi: 10.3389/fpls.2024.1335965. eCollection 2024. Front Plant Sci. 2024. PMID: 38384769 Free PMC article.
-
Heavy Metal Pollution in the Environment and Its Impact on Health: Exploring Green Technology for Remediation.Environ Health Insights. 2023 Oct 5;17:11786302231201259. doi: 10.1177/11786302231201259. eCollection 2023. Environ Health Insights. 2023. PMID: 37808962 Free PMC article. Review.
References
-
- Akram S., Najam R., Rizwani G. H., Abbas S. A. (2015). Determination of heavy metal contents by atomic absorption spectroscopy (AAS) in some medicinal plants from Pakistani and Malaysian origin. Pak. J. Pharm. Sci. 28 1781–7. - PubMed
-
- Altaf M. A., Shahid R., Ren M. X., Naz S., Altaf M. M., Qadir A., et al. (2020). Exogenous melatonin enhances salt stress tolerance in tomato seedlings. Biol. Plant 64 604–615. 10.32615/bp.2020.090 - DOI
LinkOut - more resources
Full Text Sources

