Mitochondrial Oxygenation During Cardiopulmonary Bypass: A Pilot Study
- PMID: 35924039
- PMCID: PMC9339625
- DOI: 10.3389/fmed.2022.785734
Mitochondrial Oxygenation During Cardiopulmonary Bypass: A Pilot Study
Abstract
Objective: Adequate oxygenation is essential for the preservation of organ function during cardiac surgery and cardiopulmonary bypass (CPB). Both hypoxia and hyperoxia result in undesired outcomes, and a narrow window for optimal oxygenation exists. Current perioperative monitoring techniques are not always sufficient to monitor adequate oxygenation. The non-invasive COMET® monitor could be a tool to monitor oxygenation by measuring the cutaneous mitochondrial oxygen tension (mitoPO2). This pilot study examines the feasibility of cutaneous mitoPO2 measurements during cardiothoracic procedures. Cutaneous mitoPO2 will be compared to tissue oxygenation (StO2) as measured by near-infrared spectroscopy.
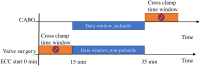
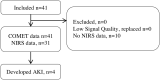
Design and method: This single-center observational study examined 41 cardiac surgery patients requiring CPB. Preoperatively, patients received a 5-aminolevulinic acid plaster on the upper arm to enable mitoPO2 measurements. After induction of anesthesia, both cutaneous mitoPO2 and StO2 were measured throughout the procedure. The patients were observed until discharge for the development of acute kidney insufficiency (AKI).
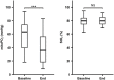
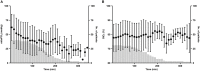
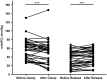
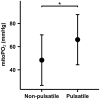
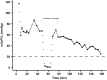
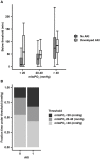
Results: Cutaneous mitoPO2 was successfully measured in all patients and was 63.5 [40.0-74.8] mmHg at the surgery start and decreased significantly (p < 0.01) to 36.4 [18.4-56.0] mmHg by the end of the CPB run. StO2 at the surgery start was 80.5 [76.8-84.3]% and did not change significantly. Cross-clamping of the aorta and the switch to non-pulsatile flow resulted in a median cutaneous mitoPO2 decrease of 7 mmHg (p < 0.01). The cessation of the aortic cross-clamping period resulted in an increase of 4 mmHg (p < 0.01). Totally, four patients developed AKI and had a lower preoperative eGFR of 52 vs. 81 ml/min in the non-AKI group. The AKI group spent 32% of the operation time with a cutaneous mitoPO2 value under 20 mmHg as compared to 8% in the non-AKI group.
Conclusion: This pilot study illustrated the feasibility of measuring cutaneous mitoPO2 using the COMET® monitor during cardiothoracic procedures. Moreover, in contrast to StO2, mitoPO2 decreased significantly with the increasing CPB run time. Cutaneous mitoPO2 also significantly decreased during the aortic cross-clamping period and increased upon the release of the clamp, but StO2 did not. This emphasized the sensitivity of cutaneous mitoPO2 to detect circulatory and microvascular changes.
Keywords: acute kidney injury; cardiopulmonary bypass and maze procedure; ischemia; microcirculation; mitochondria; mitochondrial oxygenation.
Copyright © 2022 Harms, Ubbink, de Wijs, Ligtenberg, ter Horst and Mik.
Conflict of interest statement
EM is listed as an inventor on patents related to mitochondrial oxygen measurements held by the Academic Medical Center Amsterdam and the Erasmus Medical Center Rotterdam, the Netherlands. He is the founder and shareholder of Photonics Healthcare, a company that holds exclusive licenses to these patents and that markets the COMET® system. RU is a minority shareholder of Photonics Healthcare. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Holmgaard F, Vedel AG, Rasmussen LS, Paulson OB, Nilsson JC, Ravn HB. The association between postoperative cognitive dysfunction and cerebral oximetry during cardiac surgery: a secondary analysis of a randomised trial. Br J Anaesth. (2019) 123:196–205. 10.1016/j.bja.2019.03.045 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

