Model-Informed Drug Development of New Cefoperazone Sodium and Sulbactam Sodium Combination (3:1): Pharmacokinetic/Pharmacodynamic Analysis and Antibacterial Efficacy Against Enterobacteriaceae
- PMID: 35924047
- PMCID: PMC9340253
- DOI: 10.3389/fphar.2022.856792
Model-Informed Drug Development of New Cefoperazone Sodium and Sulbactam Sodium Combination (3:1): Pharmacokinetic/Pharmacodynamic Analysis and Antibacterial Efficacy Against Enterobacteriaceae
Abstract
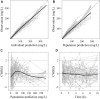
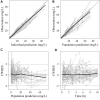
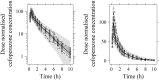
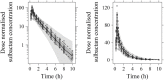
Objective: Cefoperazone/sulbactam is a commonly used antibiotic combination against the extended-spectrum beta-lactamases (ESBLs)-producing bacteria. The objective of this study was to evaluate the efficacy of a new cefoperazone/sulbactam combination (3:1) for Enterobacteriaceae infection via model-informed drug development (MIDD) approaches. Methods: Sulperazon [cefoperazone/sulbactam (2:1)] was used as a control. Pharmacokinetic (PK) data was collected from a clinical phase I trial. Minimum inhibitory concentrations (MICs) were determined using two-fold broth microdilution method. The percent time that the free drug concentration exceeded the minimum inhibitory concentration (%fT>MIC) was used as the pharmacokinetic/pharmacodynamic indicator correlated with efficacy. Models were developed to characterize the PK profile of cefoperazone and sulbactam. Monte Carlo simulations were employed to determine the investigational regimens of cefoperazone/sulbactam (3:1) for the treatment of infections caused by Enterobacteriaceae based on the probability of target attainment (PTA) against the tested bacteria. Results: Two 2-compartment models were developed to describe the PK profiles of cefoperazone and sulbactam. Simulation results following the single-dose showed that the regimens of cefoperazone/sulbactam combinations in the ratios of 3:1 and 2:1 achieved similar PTA against the tested bacteria. Simulation results from the multiple-dose showed that the dosing regimen of cefoperazone/sulbactam (4 g, TID, 3 g:1 g) showed slightly better antibacterial effect than cefoperazone/sulbactam (6 g, BID, 4 g:2 g) against the Escherichia coli (ESBL-) and Klebsiella pneumoniae (ESBL-). For the other tested bacteria, the above regimens achieved a similar PTA. Conclusions: Cefoperazone/sulbactam (3:1) showed similar bactericidal activity to sulperazon [cefoperazone/sulbactam (2:1)] against the tested bacteria. For the ESBL-producing and cefoperazone-resistant E. coli and K. pneumoniae, Cefoperazone/sulbactam (3:1) did not exhibit advantage as anticipated. Our study indicated that further clinical trials should be carried out cautiously to avoid the potential risks of not achieving the expected target.
Keywords: ESBLs; Monte Carlo simulation; PK/PD analysis; cefoperazone/sulbactam; enterobacteriaceae; model-informed drug development.
Copyright © 2022 Ji, Zhu, Li, Xue, Kuan, He, Meng, Xiang, Cui and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Pharmacokinetic and pharmacodynamic analysis of cefoperazone/sulbactam for the treatment of pediatric sepsis by Monte Carlo simulation.Anal Methods. 2022 Mar 17;14(11):1148-1154. doi: 10.1039/d1ay01385h. Anal Methods. 2022. PMID: 35225994
-
Model-Informed Drug Development, Pharmacokinetic/Pharmacodynamic Cutoff Value Determination, and Antibacterial Efficacy of Benapenem against Enterobacteriaceae.Antimicrob Agents Chemother. 2020 Feb 21;64(3):e01751-19. doi: 10.1128/AAC.01751-19. Print 2020 Feb 21. Antimicrob Agents Chemother. 2020. PMID: 31844001 Free PMC article. Clinical Trial.
-
[The reliability of using impenem, meropenem, cefoperazone-sulbactam and piperacillin-tazobactam to treat nosocomial Gram-negative bacterial infections with Monte Carlo simulation].Zhonghua Nei Ke Za Zhi. 2017 Aug 1;56(8):595-600. doi: 10.3760/cma.j.issn.0578-1426.2017.08.008. Zhonghua Nei Ke Za Zhi. 2017. PMID: 28789493 Chinese.
-
Cefoperazone/sulbactam: New composites against multiresistant gram negative bacteria?Infect Genet Evol. 2021 Mar;88:104707. doi: 10.1016/j.meegid.2021.104707. Epub 2021 Jan 5. Infect Genet Evol. 2021. PMID: 33418147 Review.
-
An Appraisal of the Pharmacokinetic and Pharmacodynamic Properties of Meropenem-Vaborbactam.Infect Dis Ther. 2020 Dec;9(4):769-784. doi: 10.1007/s40121-020-00344-z. Epub 2020 Oct 6. Infect Dis Ther. 2020. PMID: 33025557 Free PMC article. Review.
Cited by
-
Retrospective Analysis of Risk Factors for Cefoperazone/Sulbactam-Induced Thrombocytopenia in Adult Chinese Patients: A Six-Year Real-World Study.Infect Drug Resist. 2024 Sep 5;17:3901-3911. doi: 10.2147/IDR.S475590. eCollection 2024. Infect Drug Resist. 2024. PMID: 39253607 Free PMC article.
-
Development and internal validation of a model for predicting cefoperazone/sulbactam-associated coagulation disorders in Chinese inpatients.BMC Pharmacol Toxicol. 2024 Jul 12;25(1):41. doi: 10.1186/s40360-024-00761-7. BMC Pharmacol Toxicol. 2024. PMID: 38997770 Free PMC article.
-
Construction and Validation of a Nomogram Prediction Model for the Risk of Cefoperazone Sodium/Sulbactam Sodium-Related Coagulation Disorders.Infect Drug Resist. 2025 Aug 1;18:3859-3866. doi: 10.2147/IDR.S534366. eCollection 2025. Infect Drug Resist. 2025. PMID: 40766047 Free PMC article.
References
-
- Chiang T. T., Tang H. J., Chiu C. H., Chen T. L., Ho M. W., Lee C. H., et al. (2016). Antimicrobial Activities of Cefoperazone-Sulbactam in Comparison to Cefoperazone against Clinical Organisms from Medical Centers in Taiwan. J. Med. Sci. 36, 229–233.
-
- CLSI (2018). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Approved Standard-Ninth Edition. Wayne, PA: CLSI document M07-A11.
-
- CLSI (2021). Performance Standards for Antimicrobial Susceptibility Testing. 31th ed. Wayne, PA: CLSI supplement M100.
LinkOut - more resources
Full Text Sources

