Population pharmacokinetics model for escitalopram in Chinese psychiatric patients: effect of CYP2C19 and age
- PMID: 35924062
- PMCID: PMC9340256
- DOI: 10.3389/fphar.2022.964758
Population pharmacokinetics model for escitalopram in Chinese psychiatric patients: effect of CYP2C19 and age
Abstract
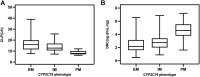
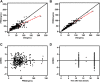
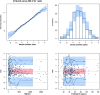
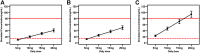
Objective: To establish a population pharmacokinetic model in Chinese psychiatric patients to characterize escitalopram pharmacokinetic profile to identify factors influencing drug exposure, and through simulation to compare the results with the established therapeutic reference range. Methods: Demographic information, dosing regimen, CYP2C19 genotype, concomitant medications, and liver and kidney function indicators were retrospectively collected for inpatients taking escitalopram with therapeutic drug monitoring from 2018 to 2021. Nonlinear mixed-effects modeling was used to model the pharmacokinetic characteristics of escitalopram. Goodness-of-fit plots, bootstrapping, and normalized prediction distribution errors were used to evaluate the model. Simulation for different dosing regimens was based on the final estimations. Results: The study comprised 106 patients and 337 measurements of serum sample. A structural model with one compartment with first-order absorption and elimination described the data adequately. The population-estimated apparent volume of distribution and apparent clearance were 815 and 16.3 L/h, respectively. Age and CYP2C19 phenotype had a significant effect on the apparent clearance (CL/F). CL/F of escitalopram decreased with increased age, and CL/F of poor metabolizer patients was significantly lower than in extensive and immediate metabolizer patients. The final model-based simulation showed that the daily dose of adolescents with poor metabolizer might be as high as 15 mg or 20 mg and referring to the therapeutic range for adults may result in overdose and a high risk of adverse effects in older patients. Conclusion: A population pharmacokinetics model of escitalopram was successfully created for the Chinese population. Depending on the age of the patients, CYP2C19 genotype and serum drug concentrations throughout treatment are required for adequate individualization of dosing regimens. When developing a regimen for older patients, especially those who are poor metabolizers, vigilance is required.
Keywords: CYP2C19 genotype; adolescent; elderly; escitalopam; population pharmacokinetics.
Copyright © 2022 Liu, Xiao, Huang, Li, Kong, Yang, Zhang, Ni, Lu, Zhang, Shang and Wen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Escitalopram and Sertraline Population Pharmacokinetic Analysis in Pediatric Patients.Clin Pharmacokinet. 2023 Nov;62(11):1621-1637. doi: 10.1007/s40262-023-01294-8. Epub 2023 Sep 27. Clin Pharmacokinet. 2023. PMID: 37755681 Free PMC article.
-
Escitalopram Personalized Dosing: A Population Pharmacokinetics Repository Method.Drug Des Devel Ther. 2023 Sep 27;17:2955-2967. doi: 10.2147/DDDT.S425654. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37789969 Free PMC article. Review.
-
Effect of age, weight, and CYP2C19 genotype on escitalopram exposure.J Clin Pharmacol. 2010 Jan;50(1):62-72. doi: 10.1177/0091270009337946. Epub 2009 Oct 19. J Clin Pharmacol. 2010. PMID: 19841156 Free PMC article. Clinical Trial.
-
Influence of C-reactive protein on the pharmacokinetics of voriconazole in relation to the CYP2C19 genotype: a population pharmacokinetics analysis.Front Pharmacol. 2024 Aug 20;15:1455721. doi: 10.3389/fphar.2024.1455721. eCollection 2024. Front Pharmacol. 2024. PMID: 39228522 Free PMC article.
-
Association of CYP2C19 and CYP2D6 Poor and Intermediate Metabolizer Status With Antidepressant and Antipsychotic Exposure: A Systematic Review and Meta-analysis.JAMA Psychiatry. 2021 Mar 1;78(3):270-280. doi: 10.1001/jamapsychiatry.2020.3643. JAMA Psychiatry. 2021. PMID: 33237321 Free PMC article.
Cited by
-
Escitalopram and Sertraline Population Pharmacokinetic Analysis in Pediatric Patients.Clin Pharmacokinet. 2023 Nov;62(11):1621-1637. doi: 10.1007/s40262-023-01294-8. Epub 2023 Sep 27. Clin Pharmacokinet. 2023. PMID: 37755681 Free PMC article.
-
Escitalopram Personalized Dosing: A Population Pharmacokinetics Repository Method.Drug Des Devel Ther. 2023 Sep 27;17:2955-2967. doi: 10.2147/DDDT.S425654. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37789969 Free PMC article. Review.
References
-
- Akil A., Bies R. R., Pollock B. G., Avramopoulos D., Devanand D. P., Mintzer J. E., et al. (2016). A population pharmacokinetic model for R- and S-citalopram and desmethylcitalopram in Alzheimer's disease patients with agitation. J. Pharmacokinet. Pharmacodyn. 43(1), 99–109. 10.1007/s10928-015-9457-6 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

