MRI Findings in Case of Post-COVID-19 Vaccination Rhabdomyolysis: A Rare Postvaccination Adverse Effect
- PMID: 35924123
- PMCID: PMC9340183
- DOI: 10.1055/s-0042-1748534
MRI Findings in Case of Post-COVID-19 Vaccination Rhabdomyolysis: A Rare Postvaccination Adverse Effect
Abstract
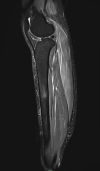
In the era of this pandemic, without any proper and efficacious availability of antiviral agents against the novel coronavirus disease 2019 (COVID-19), vaccines have come as a hope for humankind. Although adverse reactions are common after getting the COVID-19 vaccine, serious or life-threatening side effects are very uncommon in these new emergency-approved vaccines. In this case report, we describe an unusual case of adverse reaction in a patient who received the COVID-19 vaccination. The patient who received the COVID-19 vaccination presented with progressive right lower limb pain and swelling, which further progressed to bilateral shoulder pain and swelling. Ultrasonography, Doppler, and magnetic resonance imaging of right lower limb were done for the patient.
Keywords: post-COVID-19 vaccination; post-COVID-19 vaccination adverse effects; post-COVID-19 vaccination rhabdomyolysis; postvaccination rhabdomyolysis.
Indian Radiological Association. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. ( https://creativecommons.org/licenses/by-nc-nd/4.0/ ).
Conflict of interest statement
Conflicting Interest None declared.
Figures






References
-
- WHO. WHO novel coronavirus 2019 dashboard 2019https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed January 23, 2022
-
- Press Information Bureau, Government of India. Press statement by the Drugs Controller General of India (DCGI) on restricted emergency approval of COVID-19 virus vaccine 2021https://pib.gov.in/PressReleasePage.aspx?PRID=1685761. Accessed January 23, 2022 https://www.who.int/emergencies/diseases/novel-coronavirus-2019
-
- Serum Institute of India. Side effect profile of recombinant chimpanzee adenovirus vector vaccine 2021https://www.seruminstitute.com/pdf/Recombinant_Chimpanzee_Adenovirus_vec...Accessed January 23, 2022; Or Voysey M, Clemens SAC, Madhi SA, et al; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397(10269):99—111. - PMC - PubMed
-
- Oxford COVID Vaccine Trial Group Ramasamy M N, Minassian A M, Ewer K Jet al.Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial Lancet 2021396(10267)1979–1993. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

