Performance of NI-RADS on CECT Alone to Predict Recurrent Head and Neck Squamous Cell Carcinoma after Chemoradiotherapy: Added Value of RECIST 1.1
- PMID: 35924129
- PMCID: PMC9340179
- DOI: 10.1055/s-0042-1754315
Performance of NI-RADS on CECT Alone to Predict Recurrent Head and Neck Squamous Cell Carcinoma after Chemoradiotherapy: Added Value of RECIST 1.1
Abstract
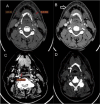
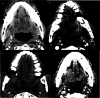
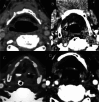
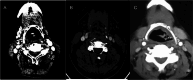
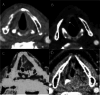
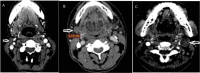
Background The Head and Neck Imaging Reporting and Data System (NI-RADS) is a standardized reporting format for the categorization of the degree of suspicion for recurrent head and neck malignancies on positron emission tomography/computed tomography. Purpose The purpose of our study was to analyze the efficacy of the NI-RADS rating scale and criteria for contrast-enhanced computed tomography (CECT) alone in predicting the local and regional recurrence of malignancies after chemoradiotherapy. Material and Methods CECT of the patients with head and neck cancers receiving radiotherapy and concurrent chemotherapy as a primary treatment was obtained 3 months after the completion of radiotherapy and NI-RADS scoring was done using components of Response Evaluation Criteria in Solid Tumors (RECIST 1.1) criteria. Their management was guided according to the recommendations based on their NI-RADS score. Results Thirty patients with squamous cell carcinoma of the neck were included in this study. The positive or negative status of the recurrent disease was based on biopsy results or follow-up protocol as recommended in NI-RADS rating scale. Fifteen patients had path proven recurrence at the primary tumor site. For primary tumor site, disease persistence rates of 4% for NI-RADS 1, 24% for NI-RADS 2, and 80% for NI-RADS 3 scores were seen. Five patients had recurrent lymph nodal disease. For lymph nodal assessment, NI-RADS categories 1, 2, and 3 revealed nodal disease recurrence rates of 5.3, 25, and 66.7%, respectively. Conclusion CECT alone may be used to assign the NI-RADS rating scale using RECIST 1.1 criteria to predict the presence or absence of recurrent tumor in patients with neck malignancies.
Keywords: CT; adults; head/neck; larynx; neoplasms-primary.
Indian Radiological Association. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. ( https://creativecommons.org/licenses/by-nc-nd/4.0/ ).
Conflict of interest statement
Conflict of Interest None.
Figures
References
-
- Kulkarni M R. Head and neck cancer burden in India. Int J Head Neck Surg. 2013;4:29–35.
-
- Garden A S, Morrison W H, Rosenthal D I, Chao K S, Ang K K. Target coverage for head and neck cancers treated with IMRT: review of clinical experiences. Semin Radiat Oncol. 2004;14(02):103–109. - PubMed
-
- Zackrisson B, Mercke C, Strander H, Wennerberg J, Cavallin-Ståhl E.A systematic overview of radiation therapy effects in head and neck cancer Acta Oncol 200342(5-6):443–461. - PubMed
-
- Paris F, Fuks Z, Kang Aet al.Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice Science 2001293(5528):293–297. - PubMed
-
- Glastonbury CM1 . Parker EE, Hoang JK. The postradiation neck: evaluating response to treatment and recognizing complications. AJR Am J Roentgenol. 2010;195:164–171. - PubMed
LinkOut - more resources
Full Text Sources

