The Predictive Value of Pretreatment Lactate Dehydrogenase and Derived Neutrophil-to-Lymphocyte Ratio in Advanced Non-Small Cell Lung Cancer Patients Treated With PD-1/PD-L1 Inhibitors: A Meta-Analysis
- PMID: 35924149
- PMCID: PMC9340347
- DOI: 10.3389/fonc.2022.791496
The Predictive Value of Pretreatment Lactate Dehydrogenase and Derived Neutrophil-to-Lymphocyte Ratio in Advanced Non-Small Cell Lung Cancer Patients Treated With PD-1/PD-L1 Inhibitors: A Meta-Analysis
Abstract
Background: The Lung Immune Prognostic Index (LIPI) combines the lactate dehydrogenase (LDH) level and the derived neutrophil-to-lymphocyte ratio (dNLR). A lot of studies have shown that LDH and dNLR are associated with the prognosis of advanced non-small cell lung cancer (NSCLC) in patients treated with programmed cell death protein 1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitors. However, previous results were inconsistent, and the conclusions remain unclear. This meta-analysis aimed to investigate the predictive value of pretreatment LDH and dNLR for NSCLC progression in patients treated with PD-1/PD-L1 inhibitors.
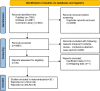
Methods: PubMed, Embase, and the Cochrane Library were searched by two researchers independently for related literature before March 2020. Hazard ratios (HRs) with 95% confidence intervals (CIs) for progression-free survival (PFS) and overall survival (OS) were extracted to assess the predictive value of LDH and dNLR. STATA 15. 0 was used to perform the meta-analysis.
Results: A total of 3,429 patients from 26 studies were included in this meta-analysis. The results revealed that high pretreatment LDH was related to poor OS (HR = 1.19, 95%CI = 1.11-1.24, p < 0.001), but not closely related to poor PFS (HR = 1.02, 95%CI = 1.00-1.04, p = 0.023 < 0.05). The pooled results for dNLR suggested that high pretreatment dNLR was related to poor OS (HR = 1.55, 95%CI = 1.33-1.80, p < 0.001) and PFS (HR = 1.33, 95%CI = 1.16-1.54, p < 0.001).
Conclusion: Both pretreatment LDH and dNLR have the potential to serve as peripheral blood biomarkers for patients with advanced NSCLC treated with PD-1/PD-L1 inhibitors. However, more studies on LDH are needed to evaluate its predictive value for PFS in patients with NSCLC.
Keywords: derived neutrophil-to-lymphocyte ratio; immunotherapy; lactate dehydrogenase; non-small cell lung cancer; prognosis.
Copyright © 2022 Zhang, Gong, Sun, Miao and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Prognostic Value of the Pretreatment Lung Immune Prognostic Index in Advanced Small Cell Lung Cancer Patients Treated With First-Line PD-1/PD-L1 Inhibitors Plus Chemotherapy.Front Oncol. 2021 Oct 8;11:697865. doi: 10.3389/fonc.2021.697865. eCollection 2021. Front Oncol. 2021. PMID: 34692478 Free PMC article.
-
Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer.JAMA Oncol. 2018 Mar 1;4(3):351-357. doi: 10.1001/jamaoncol.2017.4771. JAMA Oncol. 2018. PMID: 29327044 Free PMC article.
-
Clinical Significance of Serum Biomarkers in Stage IV Non-Small-Cell Lung Cancer Treated with PD-1 Inhibitors: LIPI Score, NLR, dNLR, LMR, and PAB.Dis Markers. 2022 Jul 30;2022:7137357. doi: 10.1155/2022/7137357. eCollection 2022. Dis Markers. 2022. PMID: 35945957 Free PMC article.
-
Prognostic value of neutrophil-lymphocyte ratio and lactate dehydrogenase in melanoma patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis.Medicine (Baltimore). 2022 Aug 12;101(32):e29536. doi: 10.1097/MD.0000000000029536. Medicine (Baltimore). 2022. PMID: 35960066 Free PMC article.
-
The relationship between blood-based tumor mutation burden level and efficacy of PD-1/PD-L1 inhibitors in advanced non-small cell lung cancer: a systematic review and meta-analysis.BMC Cancer. 2021 Nov 13;21(1):1220. doi: 10.1186/s12885-021-08924-z. BMC Cancer. 2021. PMID: 34774004 Free PMC article.
Cited by
-
Diverse Neutrophil Functions in Cancer and Promising Neutrophil-Based Cancer Therapies.Int J Mol Sci. 2022 Dec 13;23(24):15827. doi: 10.3390/ijms232415827. Int J Mol Sci. 2022. PMID: 36555469 Free PMC article. Review.
-
Development of a lung immune prognostic index-based nomogram model for predicting overall survival and immune-related adverse events in non-small cell lung cancer patients treated with sintilimab.Front Immunol. 2025 May 8;16:1569689. doi: 10.3389/fimmu.2025.1569689. eCollection 2025. Front Immunol. 2025. PMID: 40406139 Free PMC article.
-
Clinicopathological characteristics correlated with programmed cell death-ligand 1 expression in advanced lung adenocarcinoma.J Thorac Dis. 2023 Oct 31;15(10):5307-5318. doi: 10.21037/jtd-23-523. Epub 2023 Sep 11. J Thorac Dis. 2023. PMID: 37969280 Free PMC article.
-
Inflammation‑based prognostic markers of metastatic pancreatic cancer using real‑world data in Japan: The Tokushukai REAl‑world Data (TREAD) project.Oncol Lett. 2024 Jan 31;27(3):136. doi: 10.3892/ol.2024.14269. eCollection 2024 Mar. Oncol Lett. 2024. PMID: 38357476 Free PMC article.
-
Longitudinal Changes of CT-radiomic and Systemic Inflammatory Features Predict Survival in Advanced Non-Small Cell Lung Cancer Patients Treated With Immune Checkpoint Inhibitors.J Thorac Imaging. 2025 Jan 1;40(1):e0801. doi: 10.1097/RTI.0000000000000801. J Thorac Imaging. 2025. PMID: 39188157 Free PMC article.
References
-
- Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. . Pembrolizumab Versus Chemotherapy for Previously Untreated, PD-L1-Expressing, Locally Advanced or Metastatic Non-Small-Cell Lung Cancer (KEYNOTE-042): A Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet (2019) 393:1819–30. doi: 10.1016/S0140-6736(18)32409-7 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

