An autologous colonic organoid-derived monolayer model to study immune: bacterial interactions in Crohn's disease patients
- PMID: 35924188
- PMCID: PMC9342672
- DOI: 10.1002/cti2.1407
An autologous colonic organoid-derived monolayer model to study immune: bacterial interactions in Crohn's disease patients
Abstract
Objectives: Crohn's disease (CD) initiation and pathogenesis are believed to involve an environmental trigger in a genetically susceptible person that results in an immune response against commensal gut bacteria, leading to a compromised intestinal epithelial barrier and a cycle of inflammation. However, it has been difficult to study the contribution of all factors together in a physiologically relevant model and in a heterogenous patient population.
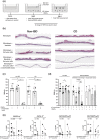
Methods: We developed an autologous colonic monolayer model that incorporated the immune response from the same donor and a commensal bacteria, Faecalibacterium prausnitzii. Two-dimensional monolayers were grown from three-dimensional organoids generated from intestinal biopsies, and the epithelial integrity of the epithelium was measured using transepithelial electrical resistance. We determined the effect of immune cells alone, bacteria alone and the co-culture of immune cells and bacteria on integrity.
Results: Monolayers derived from CD donors had impaired epithelial integrity compared to those from non-inflammatory bowel disease (IBD) donors. This integrity was further impaired by culture with bacteria, but not immune cells, despite a higher frequency of inflammatory phenotype peripheral T cells in CD donors. Variability in epithelial integrity was higher in CD donors than in non-IBD donors.
Conclusion: We have developed a new autologous model to study the complexity of CD, which allows for the comparison of the barrier properties of the colonic epithelium and the ability to study how autologous immune cells directly affect the colonic barrier and whether this is modified by luminal bacteria. This new model allows for the study of individual patients and could inform treatment decisions.
Keywords: Crohn's disease; T cells; bacteria; cytokines; epithelium; organoid.
© 2022 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Faecalibacterium prausnitzii A2-165 metabolizes host- and media-derived chemicals and induces transcriptional changes in colonic epithelium in GuMI human gut microphysiological system.Microbiome Res Rep. 2024 May 22;3(3):30. doi: 10.20517/mrr.2024.14. eCollection 2024. Microbiome Res Rep. 2024. PMID: 39421254 Free PMC article.
-
Autologous organoid co-culture model reveals T cell-driven epithelial cell death in Crohn's Disease.Front Immunol. 2022 Nov 10;13:1008456. doi: 10.3389/fimmu.2022.1008456. eCollection 2022. Front Immunol. 2022. PMID: 36439157 Free PMC article.
-
A Novel Strategy to Study the Invasive Capability of Adherent-Invasive Escherichia coli by Using Human Primary Organoid-Derived Epithelial Monolayers.Front Immunol. 2021 Mar 29;12:646906. doi: 10.3389/fimmu.2021.646906. eCollection 2021. Front Immunol. 2021. PMID: 33854511 Free PMC article.
-
[Intestinal flora and Crohn's disease].Ann Pharm Fr. 2003 Jul;61(4):276-81. Ann Pharm Fr. 2003. PMID: 12843962 Review. French.
Cited by
-
Challenges in IBD Research 2024: Preclinical Human IBD Mechanisms.Inflamm Bowel Dis. 2024 May 23;30(Suppl 2):S5-S18. doi: 10.1093/ibd/izae081. Inflamm Bowel Dis. 2024. PMID: 38778627 Free PMC article. Review.
-
Leveraging Organ-on-Chip Models to Investigate Host-Microbiota Dynamics and Targeted Therapies for Inflammatory Bowel Disease.Adv Healthc Mater. 2025 Apr;14(10):e2402756. doi: 10.1002/adhm.202402756. Epub 2024 Nov 3. Adv Healthc Mater. 2025. PMID: 39491534 Free PMC article. Review.
-
Gut-on-a-Chip Models: Current and Future Perspectives for Host-Microbial Interactions Research.Biomedicines. 2023 Feb 18;11(2):619. doi: 10.3390/biomedicines11020619. Biomedicines. 2023. PMID: 36831155 Free PMC article. Review.
-
Ion transport and epithelial barrier dysfunction in experimental models of ulcerative colitis.Am J Physiol Gastrointest Liver Physiol. 2025 Jun 1;328(6):G811-G830. doi: 10.1152/ajpgi.00204.2024. Epub 2025 Apr 4. Am J Physiol Gastrointest Liver Physiol. 2025. PMID: 40184259 Free PMC article. Review.
-
Tumour necrosis factor-α induces macromolecule translocation in HIV-derived duodenal organoids.Front Immunol. 2025 Mar 18;16:1563702. doi: 10.3389/fimmu.2025.1563702. eCollection 2025. Front Immunol. 2025. PMID: 40170863 Free PMC article.
References
-
- Uhlig HH, Powrie F. Translating immunology into therapeutic concepts for inflammatory bowel disease. Annu Rev Immunol 2018; 36: 755–781. - PubMed
-
- Kemp R, Dunn E, Schultz M. Immunomodulators in inflammatory bowel disease: an emerging role for biologic agents. BioDrugs 2013; 27: 585–590. - PubMed
-
- de Souza HSP, Fiocchi C, Iliopoulos D. The IBD interactome: an integrated view of aetiology, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol 2017; 14: 739–749. - PubMed
-
- Ben‐Horin S, Chowers Y. Loss of response to anti‐TNF treatments in Crohn's disease. Aliment Pharmacol Ther 2011; 33: 987–995. - PubMed
LinkOut - more resources
Full Text Sources
