Characterization of the early cellular immune response induced by HPV vaccines
- PMID: 35924247
- PMCID: PMC9341268
- DOI: 10.3389/fimmu.2022.863164
Characterization of the early cellular immune response induced by HPV vaccines
Abstract
Introduction: Current human papillomavirus (HPV) vaccines consist of virus-like particles (VLPs) which are based on the L1 protein, but they are produced by different expression systems and use different adjuvants. We performed in-depth immunophenotyping of multiple innate and adaptive immune cells after vaccination with bivalent versus nonavalent HPV vaccines.
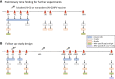
Method: Twenty pre-menopausal HPV-seronegative women were enrolled and randomized to receive three-doses of either the bivalent or the nonavalent HPV vaccine. Blood samples were collected at multiple time points from baseline up to 7 months after first vaccination. Four extensive EuroFlow flow cytometry antibody panels were used to monitor various immune cell subsets. Additionally, HPV-specific memory B- and T cells were determined by ELISPOT and HPV-specific antibody levels were measured by a VLP-based multiplex immunoassay.
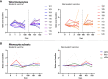
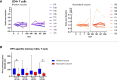
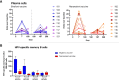
Results: In both cohorts, the numbers of plasma cells expanded in the first week after both primary and tertiary vaccination. HPV16 and HPV18-specific antibody levels and memory B and T-cell responses were higher in the bivalent than in the nonavalent vaccinees one month post third vaccination. For HPV31 and HPV45-specific antibody levels this pattern was reversed. Monocytes showed an expansion one day after vaccination in both cohorts but were significantly higher in the bivalent vaccine cohort. Large heterogeneity in responses of the other cell subsets was observed between donors.
Conclusion: This pilot study showed a consistent response of monocytes and plasma cells after vaccination and a considerable variation in other circulating immune cells in both types of HPV vaccines between donors.
Keywords: B cells; T cells; antibodies; human papillomavirus (HPV); innate cells; vaccines.
Copyright © 2022 Pasmans, Berkowska, Diks, de Mooij, Groenland, de Rond, Nicolaie, van der Burg, van Dongen, van der Klis and Buisman.
Conflict of interest statement
MB, AD, and JD report inventorship of the patent “Means and methods for multiparameter cytometry-based leukocyte subsetting” (NL2844751, PCT/NL2020/050688, priority date 5 November 2019), owned by the EuroFlow Consortium. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. We thank GlaxoSmithKline Biologicals SA for providing at cost part of the VLP’s that we used in our assays. GlaxoSmithKline Biologicals SA was provided the opportunity to review a preliminary version of this manuscript in order to ensure the protection of its proprietary information and intellectual property, but the authors are solely responsible for final content and interpretation.
Figures
Similar articles
-
Immune response following a two-dose schedule of bivalent HPV vaccination among girls and boys.Front Immunol. 2024 Jan 26;15:1327770. doi: 10.3389/fimmu.2024.1327770. eCollection 2024. Front Immunol. 2024. PMID: 38343547 Free PMC article.
-
Neutralizing and cross-neutralizing antibody titres induced by bivalent and quadrivalent human papillomavirus vaccines in the target population of organized vaccination programmes.Vaccine. 2014 Sep 15;32(41):5357-62. doi: 10.1016/j.vaccine.2014.07.014. Epub 2014 Jul 18. Vaccine. 2014. PMID: 25045814
-
Effects of varying antigens and adjuvant systems on the immunogenicity and safety of investigational tetravalent human oncogenic papillomavirus vaccines: results from two randomized trials.Vaccine. 2014 Jun 17;32(29):3694-705. doi: 10.1016/j.vaccine.2014.03.040. Epub 2014 Mar 25. Vaccine. 2014. PMID: 24674663 Clinical Trial.
-
Developments in L2-based human papillomavirus (HPV) vaccines.Virus Res. 2017 Mar 2;231:166-175. doi: 10.1016/j.virusres.2016.11.020. Epub 2016 Nov 23. Virus Res. 2017. PMID: 27889616 Free PMC article. Review.
-
[Human papillomavirus prophylactic vaccines: stakes and perspectives].Gynecol Obstet Fertil. 2006 Jul-Aug;34(7-8):647-55. doi: 10.1016/j.gyobfe.2006.05.008. Epub 2006 Jun 27. Gynecol Obstet Fertil. 2006. PMID: 16807045 Review. French.
Cited by
-
Systems serology analysis shows IgG1 and IgG3 memory responses six years after one dose of quadrivalent HPV vaccine.Nat Commun. 2025 Mar 3;16(1):2130. doi: 10.1038/s41467-025-57443-z. Nat Commun. 2025. PMID: 40032823 Free PMC article.
-
Immune response following a two-dose schedule of bivalent HPV vaccination among girls and boys.Front Immunol. 2024 Jan 26;15:1327770. doi: 10.3389/fimmu.2024.1327770. eCollection 2024. Front Immunol. 2024. PMID: 38343547 Free PMC article.
-
Performance of spectral flow cytometry and mass cytometry for the study of innate myeloid cell populations.Front Immunol. 2023 May 19;14:1191992. doi: 10.3389/fimmu.2023.1191992. eCollection 2023. Front Immunol. 2023. PMID: 37275858 Free PMC article.
-
Understanding dental hygienists' knowledge, attitudes, and practices regarding HPV vaccination.Hum Vaccin Immunother. 2025 Dec;21(1):2511484. doi: 10.1080/21645515.2025.2511484. Epub 2025 May 30. Hum Vaccin Immunother. 2025. PMID: 40444900 Free PMC article.
-
The potential use of therapeutics and prophylactic mRNA vaccines in human papillomavirus (HPV).Virol J. 2024 May 31;21(1):124. doi: 10.1186/s12985-024-02397-9. Virol J. 2024. PMID: 38822328 Free PMC article. Review.
References
-
- Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, et al. . Comparison of the Immunogenicity of the Human Papillomavirus (HPV)-16/18 Vaccine and the HPV-6/11/16/18 Vaccine for Oncogenic non-Vaccine Types HPV-31 and HPV-45 in Healthy Women Aged 18-45 Years. Hum Vaccin (2011) 7:1359–73. doi: 10.4161/hv.7.12.18282 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

