Dual-Target Multifunctional Superparamagnetic Cationic Nanoliposomes for Multimodal Imaging-Guided Synergistic Photothermal/Photodynamic Therapy of Retinoblastoma
- PMID: 35924259
- PMCID: PMC9339948
- DOI: 10.2147/IJN.S364264
Dual-Target Multifunctional Superparamagnetic Cationic Nanoliposomes for Multimodal Imaging-Guided Synergistic Photothermal/Photodynamic Therapy of Retinoblastoma
Abstract
Background: With high malignancy, retinoblastoma (RB) commonly occurs in infants and has incredible difficulty with the early diagnosis. In recent years, the integrated theranostics of multimodal imaging-guided therapy has shown promising potential for oncotherapy.
Purpose: To prepare folate/magnetic dual-target theranostic nanoparticles integrating with US/PA/MR imaging and the synergistic photothermal treatment (PTT)/photodynamic treatment (PDT) for the early diagnosis and timely intervention of RB cancer.
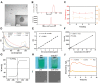
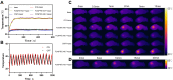
Methods: Folate/magnetic dual-target cationic nanoliposomes (CN) encapsulating indocyanine green (ICG) and perfluorohexane(PFH)(FA-CN-PFH-ICG-Fe3O4, FCNPIFE) were synthesized and characterized. Then we evaluated their targeting ability, US/PA/MR imaging effects, and the efficacy of synergistic PTT/PDT in vitro and in vivo. Finally, we explored the mechanism of synergistic PTT/PDT in Y79 tumor-bearing mice.
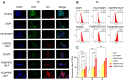
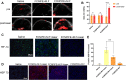
Results: FCNPIFEs were stable and uniform in 7 days. They showed excellent in vitro targeting ability with a 95.29% cell uptake rate. The in vitro US/PA/MRI imaging results of FCNPIFEs showed a concentration-dependent manner, and in vitro therapy FCNPIFEs exhibited an enhanced anticancer efficacy against Y79 cells. In vivo analysis confirmed that FCNPIFEs enabled a targeted synergistic PTT/PDT under US/PA/MR imaging guidance in Y79 tumor-bearing mice, achieving almost complete tumor regression. Immunofluorescence results displayed weaker fluorescence intensity compared with other single treatment groups, confirming that PTT/PDT synergistic therapy effect was achieved by down-regulating the expression of HIF-1α and HSP70.
Conclusion: FCNPIFEs were verified as promising theranostic nanoliposomes for RB oncotherapy and showed great potential in clinical application.
Keywords: nanomedicine; phototherapy; retinoblastoma; theranostic.
© 2022 Zheng et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures





Similar articles
-
A Dual-Model Imaging Theragnostic System Based on Mesoporous Silica Nanoparticles for Enhanced Cancer Phototherapy.Adv Healthc Mater. 2019 Oct;8(19):e1900840. doi: 10.1002/adhm.201900840. Epub 2019 Sep 12. Adv Healthc Mater. 2019. PMID: 31512403
-
Magnetic Resonance/Infrared Dual-Modal Imaging-Guided Synergistic Photothermal/Photodynamic Therapy Nanoplatform Based on Cu1.96S-Gd@FA for Precision Cancer Theranostics.J Colloid Interface Sci. 2022 Jun;615:95-109. doi: 10.1016/j.jcis.2022.01.099. Epub 2022 Jan 20. J Colloid Interface Sci. 2022. PMID: 35124510
-
Chemotherapeutic drug-photothermal agent co-self-assembling nanoparticles for near-infrared fluorescence and photoacoustic dual-modal imaging-guided chemo-photothermal synergistic therapy.J Control Release. 2017 Jul 28;258:95-107. doi: 10.1016/j.jconrel.2017.05.011. Epub 2017 May 10. J Control Release. 2017. PMID: 28501673
-
Theranostics Based on Iron Oxide and Gold Nanoparticles for Imaging- Guided Photothermal and Photodynamic Therapy of Cancer.Curr Top Med Chem. 2017;17(16):1858-1871. doi: 10.2174/1568026617666161122120537. Curr Top Med Chem. 2017. PMID: 27875977 Review.
-
Multifunctional nanotheranostics for near infrared optical imaging-guided treatment of brain tumors.Adv Drug Deliv Rev. 2022 Nov;190:114536. doi: 10.1016/j.addr.2022.114536. Epub 2022 Sep 13. Adv Drug Deliv Rev. 2022. PMID: 36108792 Review.
Cited by
-
Preclinical advance in nanoliposome-mediated photothermal therapy in liver cancer.Lipids Health Dis. 2025 Jan 31;24(1):31. doi: 10.1186/s12944-024-02429-x. Lipids Health Dis. 2025. PMID: 39891269 Free PMC article. Review.
-
Bioactive Polymeric Nanoparticles of Moringa oleifera Induced Phyto-Photothermal Sensitization for the Enhanced Therapy of Retinoblastoma.Pharmaceutics. 2023 Jan 31;15(2):475. doi: 10.3390/pharmaceutics15020475. Pharmaceutics. 2023. PMID: 36839797 Free PMC article.
-
Frontier applications of retinal nanomedicine: progress, challenges and perspectives.J Nanobiotechnology. 2025 Feb 25;23(1):143. doi: 10.1186/s12951-025-03095-6. J Nanobiotechnology. 2025. PMID: 40001147 Free PMC article. Review.
-
A multifunctional hydrogel system with synergistic effects against Staphylococcus aureus infections and inflammation in perioperative cataract surgery.Mater Today Bio. 2025 Jul 11;33:102080. doi: 10.1016/j.mtbio.2025.102080. eCollection 2025 Aug. Mater Today Bio. 2025. PMID: 40704014 Free PMC article.
-
Advancements in Nanosystems for Ocular Drug Delivery: A Focus on Pediatric Retinoblastoma.Molecules. 2024 May 11;29(10):2263. doi: 10.3390/molecules29102263. Molecules. 2024. PMID: 38792122 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

