Evaluation of Proton MR Spectroscopy for the Study of the Tongue Tissue in Healthy Subjects and Patients With Tongue Squamous Cell Carcinoma: Preliminary Findings
- PMID: 35924279
- PMCID: PMC9339644
- DOI: 10.3389/froh.2022.912803
Evaluation of Proton MR Spectroscopy for the Study of the Tongue Tissue in Healthy Subjects and Patients With Tongue Squamous Cell Carcinoma: Preliminary Findings
Abstract
Purpose: To noninvasively assess spectroscopic and metabolic profiles of healthy tongue tissue and in an exploratory objective in nontreated and treated patients with tongue squamous cell carcinoma (SCC).
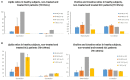
Methods: Fourteen healthy subjects (HSs), one patient with nontreated tongue SCC (NT-SCC), and two patients with treated tongue SCC (T-SCC) underwent MRI and single-voxel proton magnetic resonance spectroscopy (1H-MRS) evaluations (3 and 1.5T). Multi-echo-times 1H-MRS was performed at the medial superior part (MSP) and the anterior inferior part (AIP) of the tongue in HS, while 1H-MRS voxel was placed at the most aggressive part of the tumor for patients with tongue SCC. 1H-MRS data analysis yielded spectroscopic metabolite ratios quantified to total creatine.
Results: In HS, compared to MSP and AIP, 1H-MRS spectra revealed higher levels of creatine, a more prominent and well-identified trimethylamine-choline (TMA-Cho) peak. However, larger prominent lipid peaks were better differentiated in the tongue MSP. Compared to HS, patients with NT-SCC exhibited very high levels of lipids and relatively higher values of TMA-Cho peak. Interestingly, patients with T-SCC showed almost nonproliferation activity. However, high lipids levels were measured, although they were relatively lower than lipids levels measured in patients with NT-SCC.
Conclusion: The present study demonstrated the potential use of in-vivo 1H-MRS to noninvasively assess spectroscopic and metabolic profiles of the healthy tongue tissue in a spatial location-dependent manner. Preliminary results revealed differences between HS and patients with tongue NT-SCC as well as tongue T-SCC, which should be confirmed with more patients. 1H-MRS could be included, in the future, in the arsenal of tools for treatment response evaluation and noninvasive monitoring of patients with tongue SCC.
Keywords: 1H-MRS; noninvasive biomarkers; proton magnetic resonance spectroscopy; spectroscopic and metabolic profile; squamous cell carcinoma; tongue tissue.
Copyright © 2022 Boussida, François, Heintz, Saidak, Dakpé, Coutte, Chauffert, Devauchelle, Galmiche, Testelin, Goudot and Constans.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Preliminary study of 3T 1H MR spectroscopy in bone and soft tissue tumors.Chin Med J (Engl). 2009 Jan 5;122(1):39-43. Chin Med J (Engl). 2009. PMID: 19187615
-
Human cervical lymphadenopathy: evaluation with in vivo 1H-MRS at 1.5 T.Clin Radiol. 2005 May;60(5):592-8. doi: 10.1016/j.crad.2004.11.012. Clin Radiol. 2005. PMID: 15851048
-
Ex vivo proton MR spectroscopy (1H-MRS) for evaluation of human gastric carcinoma.Magn Reson Imaging. 2004 Jul;22(6):861-70. doi: 10.1016/j.mri.2004.01.045. Magn Reson Imaging. 2004. PMID: 15234456
-
Proton MR spectroscopy of squamous cell carcinoma of the extracranial head and neck: in vitro and in vivo studies.AJNR Am J Neuroradiol. 1997 Jun-Jul;18(6):1057-72. AJNR Am J Neuroradiol. 1997. PMID: 9194433 Free PMC article.
-
Comparison of 1.5T and 3T 1H MR spectroscopy for human brain tumors.Korean J Radiol. 2006 Jul-Sep;7(3):156-61. doi: 10.3348/kjr.2006.7.3.156. Korean J Radiol. 2006. PMID: 16969044 Free PMC article.
References
-
- Heintz A, Constans J-M. “Role of magnetic resonance spectroscopy in clinical management of brain tumors,” in Atlas of Clinical Cases on Brain Tumor Imaging, eds Özsunar, Y. Şenol, U. (Cham: Springer; ). (2020). 10.1007/978-3-030-23273-3_5 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

