Sodium-Glucose Cotransporter Inhibitors as Antidiabetic Drugs: Current Development and Future Perspectives
- PMID: 35924548
- PMCID: PMC9937539
- DOI: 10.1021/acs.jmedchem.2c00867
Sodium-Glucose Cotransporter Inhibitors as Antidiabetic Drugs: Current Development and Future Perspectives
Abstract
Sodium-glucose cotransporter 2 (SGLT-2) inhibitors (gliflozins) represent the most recently approved class of oral antidiabetic drugs. SGLT-2 overexpression in diabetic patients contributes significantly to hyperglycemia and related complications. Therefore, SGLT-2 became a highly interesting therapeutic target, culminating in the approval for clinical use of dapagliflozin and analogues in the past decade. Gliflozins improve glycemic control through a novel insulin-independent mechanism of action and, moreover, exhibit significant cardiorenal protective effects in both diabetic and nondiabetic subjects. Therefore, gliflozins have received increasing attention, prompting extensive structure-activity relationship studies and optimization approaches. The discovery that intestinal SGLT-1 inhibition can provide a novel opportunity to control hyperglycemia, through a multifactorial mechanism, recently encouraged the design of low adsorbable inhibitors selectively directed to the intestinal SGLT-1 subtype as well as of dual SGLT-1/SGLT-2 inhibitors, representing a compelling strategy to identify new antidiabetic drug candidates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
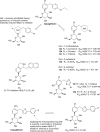

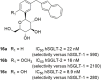
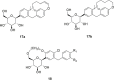
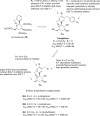
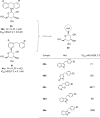
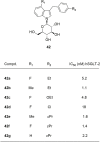


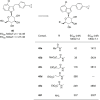
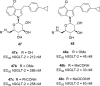
Similar articles
-
Use of Sodium-Glucose Cotransporter-2 Inhibitors in Clinical Practice for Heart Failure Prevention and Treatment: Beyond Type 2 Diabetes. A Narrative Review.Adv Ther. 2022 Feb;39(2):845-861. doi: 10.1007/s12325-021-01989-z. Epub 2021 Dec 9. Adv Ther. 2022. PMID: 34881413 Free PMC article. Review.
-
Sodium-glucose Cotransporter 2 Inhibitors: Glucose Lowering Against other Hypoglycemic Agents.Cardiovasc Hematol Disord Drug Targets. 2018;18(2):94-103. doi: 10.2174/1871529X18666180206160838. Cardiovasc Hematol Disord Drug Targets. 2018. PMID: 29412124 Review.
-
Sodium glucose cotransporter (SGLT)-2 inhibitors: Do we need them for glucose-lowering, for cardiorenal protection or both?Diabetes Obes Metab. 2019 Apr;21 Suppl 2(Suppl 2):24-33. doi: 10.1111/dom.13692. Diabetes Obes Metab. 2019. PMID: 30843294 Free PMC article. Review.
-
Sodium-glucose Cotransporter 2 Inhibitors: Nephroprotective Impact on Diabetic Kidney Disease.Cardiovasc Hematol Disord Drug Targets. 2018;18(2):120-126. doi: 10.2174/1871529X18666180206155349. Cardiovasc Hematol Disord Drug Targets. 2018. PMID: 29412122 Review.
-
Sodium-glucose cotransporter 2 (SGLT-2) inhibitors: a new antidiabetic drug class.Medchemcomm. 2018 Jun 6;9(8):1273-1281. doi: 10.1039/c8md00183a. eCollection 2018 Aug 1. Medchemcomm. 2018. PMID: 30151080 Free PMC article. Review.
Cited by
-
Standardized Chromatographic and Computational Approaches for Lipophilicity Analysis of Five Gliflozin Antidiabetic Drugs in Relation to Their Biological Activity.Molecules. 2024 Dec 31;30(1):115. doi: 10.3390/molecules30010115. Molecules. 2024. PMID: 39795172 Free PMC article.
-
Application of SGLT-2 inhibitors in non-diabetic CKD: mechanisms, efficacy, and safety.Front Med (Lausanne). 2025 Jul 1;12:1574693. doi: 10.3389/fmed.2025.1574693. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40665981 Free PMC article. Review.
-
The association between hypertension and different types of dietary carbohydrates.Cardiovasc Endocrinol Metab. 2024 Nov 14;13(4):e00317. doi: 10.1097/XCE.0000000000000317. eCollection 2024 Dec. Cardiovasc Endocrinol Metab. 2024. PMID: 40099269 Free PMC article.
-
Transport and inhibition mechanism of the human SGLT2-MAP17 glucose transporter.Nat Struct Mol Biol. 2024 Jan;31(1):159-169. doi: 10.1038/s41594-023-01134-0. Epub 2023 Dec 6. Nat Struct Mol Biol. 2024. PMID: 38057552 Free PMC article.
-
Comprehensive Cardiovascular and Renal Protection in Patients with Type 2 Diabetes.J Clin Med. 2023 Jun 8;12(12):3925. doi: 10.3390/jcm12123925. J Clin Med. 2023. PMID: 37373620 Free PMC article. Review.
References
-
- Sun H.; Saeedi P.; Karuranga S.; Pinkepank M.; Ogurtsova K.; Duncan B. B.; Stein C.; Basit A.; Chan J. C. N.; Mbanya J. C.; Pavkov M. E.; Ramachandaran A.; Wild S. H.; James S.; Herman W. H.; Zhang P.; Bommer C.; Kuo S.; Boyko E. J.; Magliano D. J. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119.10.1016/j.diabres.2021.109119. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Chemical Information
Medical
Miscellaneous

