Conducting clinical research in the era of the COVID-19 pandemic: Challenges and lessons for Speech-Language Pathology and Audiology research
- PMID: 35924604
- PMCID: PMC9350197
- DOI: 10.4102/sajcd.v69i2.898
Conducting clinical research in the era of the COVID-19 pandemic: Challenges and lessons for Speech-Language Pathology and Audiology research
Erratum in
-
Erratum: Conducting clinical research in the era of the COVID-19 pandemic: Challenges and lessons for Speech-Language Pathology and Audiology research.S Afr J Commun Disord. 2023 May 23;70(1):942. doi: 10.4102/sajcd.v70i1.942. S Afr J Commun Disord. 2023. PMID: 37265166 Free PMC article. No abstract available.
Abstract
Background: The novel coronavirus disease 2019 (COVID-19) presented new and unanticipated challenges to the academic training and performance of clinical research at undergraduate and postgraduate levels of training. This highlighted the need for reimagining research designs and methods to ensure continued generation of knowledge - a core function of a research-intensive university. Whilst adhering to government regulations geared towards protecting both the research participants and researchers, innovative research methods are required.
Objective: The purpose of this scoping review is to explore published evidence on innovative clinical research methods and processes employed during COVID-19 and to document challenges encountered and lessons that the fields of Speech-Language Pathology and Audiology can learn.
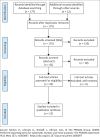
Methods: Electronic bibliographic databases including Science Direct, PubMed, Scopus, MEDLINE, ProQuest were searched to identify peer-reviewed publications, published in English, between 2019 and 2021, related to innovative clinical research methods and processes applied where in-person contact is regulated.
Results: Significant challenges with conducting research in the COVID-19 era were identified, with important lessons learned and numerous opportunities that have relevance for this pandemic era and beyond. These findings are presented under 10 themes that emerged that highlight important considerations for research methods and processes during a pandemic and beyond. The findings of this study also raise implications for telehealth from which low- and middle-income countries (LMICs), where resource challenges exist, can benefit.
Conclusion: Challenges and opportunities identified in this review have relevance for the field of Speech-Language Pathology and Audiology as far as current and future (beyond COVID-19) clinical research planning is concerned.
Keywords: COVID-19; South Africa; Speech-Language Pathology; audiology; challenges; clinical research; ethical considerations; lessons.
Conflict of interest statement
The authors declare that they have no financial or personal relationships which may have inappropriately influenced them in writing this article.
Figures
References
-
- Arksey, H., & O’Malley, L. (2005). Scoping studies: Towards a methodological framework. International Journal of Social Research Methodology: Theory and Practice, 8(1), 19–32. 10.1080/1364557032000119616 - DOI
-
- Bailey, C., Black, J.R.M., & Swanton, C. (2020). Cancer research: The lessons to learn from COVID-19. Cancer Discovery, 10(9), 1263–1266. 10.1158/2159-8290.Cd-20-0823 - DOI - PubMed
-
- Beach, M.C., Lederman, H.M., Singleton, M., Brower, R.G., Carrese, J., Ford, D.E., … Zenilman, J.M. (2020). Desperate times: Protecting the public from research without consent or oversight during public health emergencies. Annals of Internal Medicine, 173(11), 926–928. 10.7326/M20-4631 - DOI - PMC - PubMed
-
- Bookman, R.J., Cimino, J.J., Harle, C.A., Kost, R.G., Mooney, S., Pfaff, E., … Tsinoremas, N.F. (2021). Research informatics and the COVID-19 pandemic: Challenges, innovations, lessons learned, and recommendations. Journal of Clinical and Translational Science, 5(1), e110. 10.1017/cts.2021.26 - DOI - PMC - PubMed
-
- Cagnazzo, C., Besse, M.G., Manfellotto, D., Minghetti, P., Cazzaniga, S., Cottini, L., … Gussoni, G. (2021). Lessons learned from COVID-19 for clinical research operations in Italy: What have we learned and what can we apply in the future? [Editorial]. Tumori, 107(1), 6–11. 10.1177/0300891620977916 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical