High bone mass in mice can be linked to lower osteoclast formation, resorptive capacity, and restricted in vitro sensitivity to inhibition by stable sulforaphane
- PMID: 35924674
- PMCID: PMC9804804
- DOI: 10.1002/cbf.3734
High bone mass in mice can be linked to lower osteoclast formation, resorptive capacity, and restricted in vitro sensitivity to inhibition by stable sulforaphane
Abstract
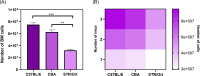
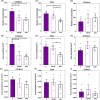
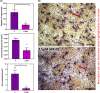
Mouse strains can have divergent basal bone mass, yet this phenotype is seldom reflected in the design of studies seeking to identify new modulators of bone resorption by osteoclasts. Sulforaphane exerts inhibitory effects on in vitro osteoclastogenesis in cells from C57BL/6 mice. Here, we explore whether a divergent basal bone mass in different mouse strains is linked both to in vitro osteoclastogenic potential and to SFX-01 sensitivity. Accordingly, osteoclasts isolated from the bone marrow (BM) of C57BL/6, STR/Ort and CBA mice with low, high, and intermediate bone mass, respectively, were cultured under conditions to promote osteoclast differentiation and resorption; they were also treated with chemically stabilised sulforaphane (SFX-01) and respective sensitivity to inhibition evaluated by counting osteoclast number/resorption activity on dentine discs. We observed that osteoclastogenesis exhibited different macrophage colony-stimulating factor/receptor activator of nuclear factor kappa-Β ligand sensitivity in these mouse strains, with cells from C57BL/6 and CBA generating higher osteoclast numbers than STR/Ort; the latter formed only half as many mature osteoclasts. We found that 100 nM SFX-01 exerted a potent and significant reduction in osteoclast number and resorptive activity in cells derived from C57BL/6 mice. In contrast, 10-fold higher SFX-01 concentrations were required for similar inhibition in CBA-derived cells and, strikingly, a further 2.5-fold greater concentration was required for significant restriction of osteoclast formation/function in STR/Ort. These data are consistent with the notion that the BM osteoclast precursor population contributes to the relative differences in mouse bone mass and that mice with higher bone mass exhibit lower in vitro osteoclastogenic potential as well as reduced sensitivity to inhibition by SFX-01.
Keywords: SFX-01; STR/Ort mice; bone mass; osteoclastogenesis; osteoclasts; sulforaphane.
© 2022 The Authors. Cell Biochemistry and Function published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Stable Sulforaphane Targets the Early Stages of Osteoclast Formation to Engender a Lasting Functional Blockade of Osteoclastogenesis.Cells. 2024 Jan 16;13(2):165. doi: 10.3390/cells13020165. Cells. 2024. PMID: 38247857 Free PMC article.
-
BSP and RANKL induce osteoclastogenesis and bone resorption synergistically.J Bone Miner Res. 2005 Sep;20(9):1669-79. doi: 10.1359/JBMR.050511. Epub 2005 May 16. J Bone Miner Res. 2005. PMID: 16059638
-
Stable sulforaphane protects against gait anomalies and modifies bone microarchitecture in the spontaneous STR/Ort model of osteoarthritis.Bone. 2017 Oct;103:308-317. doi: 10.1016/j.bone.2017.07.028. Epub 2017 Aug 1. Bone. 2017. PMID: 28778596 Free PMC article.
-
Saurolactam inhibits osteoclast differentiation and stimulates apoptosis of mature osteoclasts.J Cell Physiol. 2009 Dec;221(3):618-28. doi: 10.1002/jcp.21892. J Cell Physiol. 2009. PMID: 19653230
-
Distinct osteoclast precursors in the bone marrow and extramedullary organs characterized by responsiveness to Toll-like receptor ligands and TNF-alpha.J Immunol. 2003 Nov 15;171(10):5130-9. doi: 10.4049/jimmunol.171.10.5130. J Immunol. 2003. PMID: 14607912
Cited by
-
Bone marrow lesions: plugging the holes in our knowledge using animal models.Nat Rev Rheumatol. 2023 Jul;19(7):429-445. doi: 10.1038/s41584-023-00971-z. Epub 2023 May 24. Nat Rev Rheumatol. 2023. PMID: 37225964 Review.
-
Stable Sulforaphane Targets the Early Stages of Osteoclast Formation to Engender a Lasting Functional Blockade of Osteoclastogenesis.Cells. 2024 Jan 16;13(2):165. doi: 10.3390/cells13020165. Cells. 2024. PMID: 38247857 Free PMC article.
References
-
- Väänänen HK, Liu Y, Lehenkari P, Uemara T. How do osteoclasts resorb bone? Materials Sci Eng. 1998;6(4):205‐209.
-
- de Vernejoul MC, Kornak U. Heritable sclerosing bone disorders: presentation and new molecular mechanisms. Ann N Y Acad Sci. 2010;1192:269‐277. - PubMed
-
- Orriss IR, Arnett TR. Rodent osteoclast cultures. Methods Mol Biol. 2012;816:103‐117. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

