The impact of maternal-fetal omalizumab transfer on peanut-specific responses in an ex vivo placental perfusion model
- PMID: 35924691
- PMCID: PMC10087130
- DOI: 10.1111/all.15468
The impact of maternal-fetal omalizumab transfer on peanut-specific responses in an ex vivo placental perfusion model
Conflict of interest statement
AK, BH, JSL, BP, KS, and CW have nothing to disclose. ZS holds advisory board roles for Nutricia/Danone, Aimmune, and Sanofi. TE reports to act as local PI for company‐sponsored trials by DBV and sub‐investigator for Regeneron, holds grants from Innovation Fund Denmark, CIHR outside the submitted work. He is Co‐Investigator or scientific lead in three investigator‐initiated oral immunotherapy trials supported by the Food Allergy and Anaphylaxis Program SickKids and serves as an associate editor for Allergy. He/his laboratory received unconditional/in‐kind contributions from Macro Array Diagnostics and an unrestricted grant from ALK. He holds advisory board roles for ALK, Nutricia/Danone, and Aimmune.
Figures
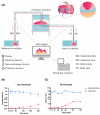
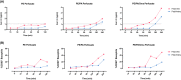
References
-
- Urbaniak SJ, Duncan JI, Armstrong‐Fisher SS, Abramovich DR, Page KR. Transfer of anti‐D antibodies across the isolated perfused human placental lobule and inhibition by high‐dose intravenous immunoglobulin: a possible mechanism of action. Br J Haematol. 1997;96(1):186‐193. doi: 10.1046/J.1365-2141.1997.8762507.X - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

