Soluble Urokinase Plasminogen Activator Receptor and Venous Thromboembolism in COVID-19
- PMID: 35924778
- PMCID: PMC9683642
- DOI: 10.1161/JAHA.122.025198
Soluble Urokinase Plasminogen Activator Receptor and Venous Thromboembolism in COVID-19
Abstract
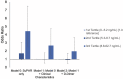
Background Venous thromboembolism (VTE) contributes significantly to COVID-19 morbidity and mortality. The urokinase receptor system is involved in the regulation of coagulation. Levels of soluble urokinase plasminogen activator receptor (suPAR) reflect hyperinflammation and are strongly predictive of outcomes in COVID-19. Whether suPAR levels identify patients with COVID-19 at risk for VTE is unclear. Methods and Results We leveraged a multinational observational study of patients hospitalized for COVID-19 with suPAR and D-dimer levels measured on admission. In 1960 patients (mean age, 58 years; 57% men; 20% Black race), we assessed the association between suPAR and incident VTE (defined as pulmonary embolism or deep vein thrombosis) using logistic regression and Fine-Gray modeling, accounting for the competing risk of death. VTE occurred in 163 (8%) patients and was associated with higher suPAR and D-dimer levels. There was a positive association between suPAR and D-dimer (β=7.34; P=0.002). Adjusted for clinical covariables, including D-dimer, the odds of VTE were 168% higher comparing the third with first suPAR tertiles (adjusted odds ratio, 2.68 [95% CI, 1.51-4.75]; P<0.001). Findings were consistent when stratified by D-dimer levels and in survival analysis accounting for death as a competing risk. On the basis of predicted probabilities from random forest, a decision tree found the combined D-dimer <1 mg/L and suPAR <11 ng/mL cutoffs, identifying 41% of patients with only 3.6% VTE probability. Conclusions Higher suPAR was associated with incident VTE independently of D-dimer in patients hospitalized for COVID-19. Combining suPAR and D-dimer identified patients at low VTE risk. Registration URL: https://www.clinicaltrials.gov; Unique identifier: NCT04818866.
Keywords: COVID‐19; soluble urokinase plasminogen activator receptor; thromboembolism.
Figures


References
-
- Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, Fragkou A, Rapti A, Damoulari C, Fantoni M, et al. Early treatment of COVID‐19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double‐blind, randomized controlled phase 3 trial. Nat Med. 2021;27:1752–1760. doi: 10.1038/s41591-021-01499-z - DOI - PMC - PubMed
-
- Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, et al. Autopsy findings and venous thromboembolism in patients with COVID‐19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

