Ruminal Microbiota Determines the High-Fiber Utilization of Ruminants: Evidence from the Ruminal Microbiota Transplant
- PMID: 35924933
- PMCID: PMC9430676
- DOI: 10.1128/spectrum.00446-22
Ruminal Microbiota Determines the High-Fiber Utilization of Ruminants: Evidence from the Ruminal Microbiota Transplant
Abstract
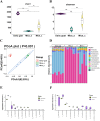
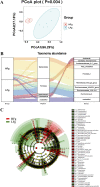
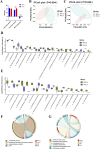
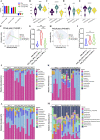
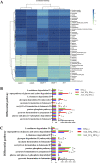
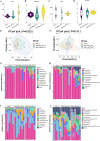
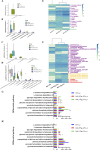
The rumen, which contains a series of prokaryotes and eukaryotes with high abundance, determines the high ability to degrade complex carbohydrates in ruminants. Using 16S rRNA gene sequencing, we compared the ruminal microbiota of dairy goats with that in the foregut and colon of mice and found more Bacteroides identified in the rumen, which helps ruminants to utilize plant-derived polysaccharides, cellulose, and other structural carbohydrates. Furthermore, high-fiber diets did not significantly increase intestinal fiber-degrading bacteria in mice, but did produce higher levels of ruminal fiber-degrading bacteria in dairy goats. Through rumen microbe transplantation (RMT), we found that rumen-derived fiber-degrading bacteria can colonize the intestines of mice to exert their fiber-degrading function, but their colonization efficiency is affected by diet. Additionally, the colonization of these fiber-degrading bacteria in the colon may involve higher content of butyrate in the colon, protecting the colonic epithelial barrier and promoting energy metabolism. Overall, the fiber degradation function of rumen bacteria through RMT was verified, and our results provide new insights into isolating the functional and beneficial fiber-degrading bacteria in the rumen, providing a theoretical basis for the role of dietary fiber in intestinal health. IMPORTANCE Ruminants have a powerful progastric digestive system that converts structural carbohydrates into nutrients useful to humans. It is well known that this phenomenon is due to the fact that the rumen of ruminants is a natural microbial fermenter, which can ferment structural carbohydrates such as cellulose and hemicellulose and transform them into volatile fatty acids to supply energy for host. However, monogastric animals have an inherent disadvantage in utilizing fiber, so screening rumen-derived fiber-degrading bacteria as a fermentation strain for biological feed is needed in an attempt at improving the fiber digestibility of monogastric animals. In this study, a ruminal microbiota transplant experiment from goats to mice proves that ruminal microbiota could serve as a key factor in utilization of high-fiber diets and provides a new perspective for the development of probiotics with fiber degradation function from the rumen and the importance of the use of prebiotics during the intake of probiotics.
Keywords: dairy goats; fiber-degrading bacteria; high-fiber diet; mice; rumen microbe transplantation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

