Triosephosphate Isomerase and Its Product Glyceraldehyde-3-Phosphate Are Involved in the Regulatory Mechanism That Suppresses Exit from the Quiescent State in Yeast Cells
- PMID: 35924934
- PMCID: PMC9430402
- DOI: 10.1128/spectrum.00897-22
Triosephosphate Isomerase and Its Product Glyceraldehyde-3-Phosphate Are Involved in the Regulatory Mechanism That Suppresses Exit from the Quiescent State in Yeast Cells
Abstract
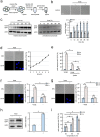
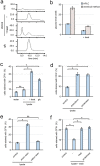
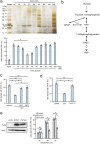
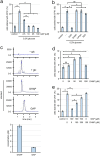
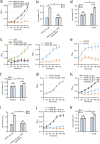
Cells of the budding yeast Saccharomyces cerevisiae form spores or stationary cells upon nutrient starvation. These quiescent cells are known to resume mitotic growth in response to nutrient signals, but the mechanism remains elusive. Here, we report that quiescent yeast cells are equipped with a negative regulatory mechanism which suppresses the commencement of mitotic growth. The regulatory process involves a glycolytic enzyme, triosephosphate isomerase (Tpi1), and its product, glyceraldehyde-3-phosphate (GAP). GAP serves as an inhibitory signaling molecule; indeed, the return to growth of spores or stationary cells is suppressed by the addition of GAP even in nutrient-rich growth media, though mitotic cells are not affected. Reciprocally, dormancy is abolished by heat treatment because of the heat sensitivity of Tpi1. For example, spores commence germination merely upon heat treatment, which indicates that the negative regulatory mechanism is actively required for spores to prevent premature germination. Stationary cells of Candida glabrata are also manipulated by heat and GAP, suggesting that the regulatory process is conserved in the pathogenic yeast. IMPORTANCE Our results suggest that, in quiescent cells, nutrient signals do not merely provoke a positive regulatory process to commence mitotic growth. Exit from the quiescent state in yeast cells is regulated by balancing between the positive and negative signaling pathways. Identifying the negative regulatory pathway would provide new insight into the regulation of the transition from the quiescent to the mitotic state. Clinically, quiescent cells are problematic because they are resistant to environmental stresses and antibiotics. Given that the quiescent state is modulated by manipulation of the negative regulatory mechanism, understanding this process is important not only for its biological interest but also as a potential target for antifungal treatment.
Keywords: glyceraldehyde-3-phosphate; quiescent cells; spores; triosephosphate isomerase; yeasts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Triosephosphate isomerase: removal of a putatively electrophilic histidine residue results in a subtle change in catalytic mechanism.Biochemistry. 1988 Aug 9;27(16):5948-60. doi: 10.1021/bi00416a019. Biochemistry. 1988. PMID: 2847777
-
Reaction of triosephosphate isomerase with L-glyceraldehyde 3-phosphate and triose 1,2-enediol 3-phosphate.Biochemistry. 1985 Feb 12;24(4):949-53. doi: 10.1021/bi00325a021. Biochemistry. 1985. PMID: 3995002
-
Substrate product equilibrium on a reversible enzyme, triosephosphate isomerase.Proc Natl Acad Sci U S A. 2007 Feb 13;104(7):2080-5. doi: 10.1073/pnas.0608876104. Epub 2007 Feb 7. Proc Natl Acad Sci U S A. 2007. PMID: 17287353 Free PMC article.
-
Medical and Veterinary Importance of the Moonlighting Functions of Triosephosphate Isomerase.Curr Protein Pept Sci. 2019;20(4):304-315. doi: 10.2174/1389203719666181026170751. Curr Protein Pept Sci. 2019. PMID: 30370845 Review.
-
On the three-dimensional structure and catalytic mechanism of triose phosphate isomerase.Philos Trans R Soc Lond B Biol Sci. 1981 Jun 26;293(1063):159-71. doi: 10.1098/rstb.1981.0069. Philos Trans R Soc Lond B Biol Sci. 1981. PMID: 6115415 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

