Characterization of the (Engineered) Branching Sucrase GtfZ-CD2 from Apilactobacillus kunkeei for Efficient Glucosylation of Benzenediol Compounds
- PMID: 35924943
- PMCID: PMC9397098
- DOI: 10.1128/aem.01031-22
Characterization of the (Engineered) Branching Sucrase GtfZ-CD2 from Apilactobacillus kunkeei for Efficient Glucosylation of Benzenediol Compounds
Abstract
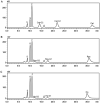
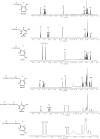
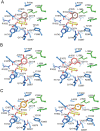
Branching sucrases, a subfamily of Glycoside Hydrolase family (GH70), display transglycosidase activity using sucrose as donor substrate to catalyze glucosylation reaction in the presence of suitable acceptor substrates. In this study, the (α1→3) branching sucrase GtfZ-CD2 from Apilactobacillus kunkeei DSM 12361 was demonstrated to glucosylate benzenediol compounds (i.e., catechol, resorcinol, and hydroquinone) to form monoglucoside and diglucoside products. The production and yield of catechol glucosylated products were significantly higher than that of resorcinol and hydroquinone, revealing a preference for adjacent aromatic hydroxyl groups in glucosylation. Amino residues around acceptor substrate binding subsite +1 were targeted for semirational mutagenesis, yielding GtfZ-CD2 variants with improved resorcinol and hydroquinone glucosylation. Mutant L1560Y with improved hydroquinone mono-glucosylated product synthesis allowed enzymatic conversion of hydroquinone into α-arbutin. This study thus revealed the high potential of GH70 branching sucrases for glucosylating noncarbohydrate molecules. IMPORTANCE Glycosylation represents one of the most important ways to expand the diversity of natural products and improve their physico-chemical properties. Aromatic polyphenol compounds widely found in plants are reported to exhibit various remarkable biological activities; however, they generally suffer from low solubility and stability, which can be improved by glycosylation. Our present study on the glucosylation of benzenediol compounds by GH70 branching sucrase GtfZ-CD2 and its semirational engineering to improve the glucosylation efficiency provides insight into the mechanism of acceptor substrates binding and its glucosylation selectivity. The results demonstrate the potential of using branching sucrase as an effective enzymatic glucosylation tool.
Keywords: GH70; benzenediol; branching sucrase; glucosylation; α-arbutin.
Conflict of interest statement
The authors declare a conflict of interest. L.B. is employed by CarbExplore Research BV, a company that produces branching sucrases, glucansucrases, and their products.
Figures
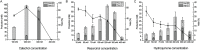
Similar articles
-
Biochemical characterization of a GH70 protein from Lactobacillus kunkeei DSM 12361 with two catalytic domains involving branching sucrase activity.Appl Microbiol Biotechnol. 2018 Sep;102(18):7935-7950. doi: 10.1007/s00253-018-9236-6. Epub 2018 Jul 24. Appl Microbiol Biotechnol. 2018. PMID: 30043269
-
Enzymatic glucosylation of polyphenols using glucansucrases and branching sucrases of glycoside hydrolase family 70.Crit Rev Food Sci Nutr. 2023;63(21):5247-5267. doi: 10.1080/10408398.2021.2016598. Epub 2021 Dec 15. Crit Rev Food Sci Nutr. 2023. PMID: 34907830 Review.
-
Engineering a branching sucrase for flavonoid glucoside diversification.Sci Rep. 2018 Oct 11;8(1):15153. doi: 10.1038/s41598-018-33394-y. Sci Rep. 2018. PMID: 30310109 Free PMC article.
-
Development of Slowly Digestible Starch Derived α-Glucans with 4,6-α-Glucanotransferase and Branching Sucrase Enzymes.J Agric Food Chem. 2020 Jun 17;68(24):6664-6671. doi: 10.1021/acs.jafc.0c01465. Epub 2020 Jun 8. J Agric Food Chem. 2020. PMID: 32437608 Free PMC article.
-
GH13 amylosucrases and GH70 branching sucrases, atypical enzymes in their respective families.Cell Mol Life Sci. 2016 Jul;73(14):2661-79. doi: 10.1007/s00018-016-2244-8. Epub 2016 May 3. Cell Mol Life Sci. 2016. PMID: 27141938 Free PMC article. Review.
Cited by
-
Semi-rational engineering of an α-L-fucosidase for regioselective synthesis of fucosyl-N-acetylglucosamine disaccharides.Food Chem (Oxf). 2025 Feb 11;10:100244. doi: 10.1016/j.fochms.2025.100244. eCollection 2025 Jun. Food Chem (Oxf). 2025. PMID: 40034538 Free PMC article.
References
-
- Leemhuis H, Pijning T, Dobruchowska JM, van Leeuwen SS, Kralj S, Dijkstra BW, Dijkhuizen L. 2013. Glucansucrases: three-dimensional structures, reactions, mechanism, α-glucan analysis and their implications in biotechnology and food applications. J Biotechnol 163:250–272. 10.1016/j.jbiotec.2012.06.037. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

