Neurotrophin Pathway Receptors NGFR and TrkA Control Perineural Invasion, Metastasis, and Pain in Oral Cancer
- PMID: 35925599
- PMCID: PMC9533666
- DOI: 10.1002/adbi.202200190
Neurotrophin Pathway Receptors NGFR and TrkA Control Perineural Invasion, Metastasis, and Pain in Oral Cancer
Abstract
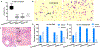
Oral squamous cell carcinoma (OSCC) patients suffer from poor survival due to metastasis or locoregional recurrence, processes that are both facilitated by perineural invasion (PNI). OSCC has higher rates of PNI than other cancer subtypes, with PNI present in 80% of tumors. Despite the impact of PNI on oral cancer prognosis and pain, little is known about the genes that drive PNI, which in turn drive pain, invasion, and metastasis. In this study, clinical data, preclinical, and in vitro models are leveraged to elucidate the role of neurotrophins in OSCC metastasis, PNI, and pain. The expression data in OSCC patients with metastasis, PNI, or pain demonstrate dysregulation of neurotrophin genes. TrkA and nerve growth factor receptor (NGFR) are focused, two receptors that are activated by NGF, a neurotrophin expressed at high levels in OSCC. It is demonstrated that targeted knockdown of these two receptors inhibits proliferation and invasion in an in vitro and preclinical model of OSCC, and metastasis, PNI, and pain. It is further determined that TrkA knockdown alone inhibits thermal hyperalgesia, whereas NGFR knockdown alone inhibits mechanical allodynia. Collectively the results highlight the ability of OSCC to co-opt different components of the neurotrophin pathway in metastasis, PNI, and pain.
Keywords: cancer pain; metastasis; neurotrophin; oral cancer; perineural invasion.
© 2022 The Authors. Advanced Biology published by Wiley-VCH GmbH.
Figures

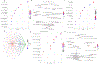

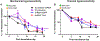
References
-
- Bapat AA, Hostetter G, Von Hoff DD, and Han H, Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer, 2011. 11(10): p. 695–707. - PubMed
-
- Connelly ST and Schmidt BL, Evaluation of pain in patients with oral squamous cell carcinoma. J Pain, 2004. 5(9): p. 505–10. - PubMed
-
- Hammerlid E, Bjordal K, Ahlner-Elmqvist M, Boysen M, Evensen JF, Biorklund A, Jannert M, Kaasa S, Sullivan M, and Westin T, A prospective study of quality of life in head and neck cancer patients. Part I: at diagnosis. Laryngoscope, 2001. 111(4 Pt 1): p. 669–80. - PubMed
-
- Kolokythas A, Cox DP, Dekker N, and Schmidt BL, Nerve Growth Factor and Tyrosine Kinase A Receptor in Oral Squamous Cell Carcinoma: Is There an Association With Perineural Invasion? Journal of Oral and Maxillofacial Surgery, 2010. 68(6): p. 1290–1295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

