Household Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 From Adult Index Cases With and Without Human Immunodeficiency Virus in South Africa, 2020-2021: A Case-Ascertained, Prospective, Observational Household Transmission Study
- PMID: 35925613
- PMCID: PMC9384657
- DOI: 10.1093/cid/ciac640
Household Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 From Adult Index Cases With and Without Human Immunodeficiency Virus in South Africa, 2020-2021: A Case-Ascertained, Prospective, Observational Household Transmission Study
Abstract
Background: In South Africa, 19% of adults are living with human immunodeficiency virus (HIV; LWH). Few data on the influence of HIV on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) household transmission are available.
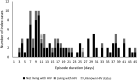
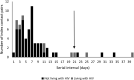
Methods: We performed a case-ascertained, prospective household transmission study of symptomatic adult index SARS-CoV-2 cases LWH and not living with HIV (NLWH) and their contacts from October 2020 to September 2021. Households were followed up 3 times a week for 6 weeks to collect nasal swabs for SARS-CoV-2 testing. We estimated household cumulative infection risk (HCIR) and duration of SARS-CoV-2 positivity (at a cycle threshold value <30 as proxy for high viral load).
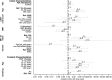
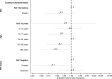
Results: HCIR was 59% (220 of 373), not differing by index HIV status (60% LWH vs 58% NLWH). HCIR increased with index case age (35-59 years: adjusted OR [aOR], 3.4; 95% CI, 1.5-7.8 and ≥60 years: aOR, 3.1; 95% CI, 1.0-10.1) compared with 18-34 years and with contacts' age, 13-17 years (aOR, 7.1; 95% CI, 1.5-33.9) and 18-34 years (aOR, 4.4; 95% CI, 1.0-18.4) compared with <5 years. Mean positivity was longer in cases LWH (adjusted hazard ratio, 0.4; 95% CI, .1-.9).
Conclusions: Index HIV status was not associated with higher HCIR, but cases LWH had longer positivity duration. Adults aged >35 years were more likely to transmit and individuals aged 13-34 to be infected SARS-CoV-2 in the household. As HIV infection may increase transmission, health services must maintain HIV testing and antiretroviral therapy initiation.
Keywords: COVID-19; HIV; SARS-CoV-2; acquisition; transmission.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. C. C. has received grant support from Sanofi Pasteur, the CDC, Wellcome Trust, Programme for Applied Technologies in Health, Bill & Melinda Gates Foundation, and South African Medical Research Council. N. W., A. v. G., J. K., and S. W. report receiving grant funds paid to institution from Sanofi Pasteur and the Bill & Melinda Gates Foundation. A. v. G. also reports an unpaid position as chairperson of the National Advisory Group on Immunisation. L. L. reports receiving grant funds from the CDC and Bill & Melinda Gates Foundation. N. M. reports receiving grant funds paid to institution from Pfizer to conduct observational studies on the burden of pneumonia in South Africa; unpaid participation on a data and safety monitoring board of a tuberculosis (TB) host-directed therapies trial and a scientific advisory board on a trial of an electronic reminder to take daily TB treatment; and an unpaid leadership or fiduciary role with the Setshaba Research Center. S. W. reports an unpaid leadership or fiduciary role for a board, society, committee, or advocacy group. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- Africa CDC . Africa CDC COVID-19 Dashboard. Available at: https://africacdc.org/covid-19/. Accessed 25 January 2022.
-
- UNAIDS . Available at: https://www.unaids.org/sites/default/files/media_asset/2020_aids-data-bo.... UNAIDS Data 2020. Accessed 23 January 2022.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

