The Single Residue K12 Governs the Exceptional Voltage Sensitivity of Mitochondrial Voltage-Dependent Anion Channel Gating
- PMID: 35925797
- PMCID: PMC12293989
- DOI: 10.1021/jacs.2c03316
The Single Residue K12 Governs the Exceptional Voltage Sensitivity of Mitochondrial Voltage-Dependent Anion Channel Gating
Abstract
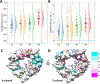
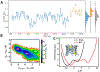
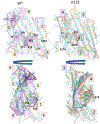
The voltage-dependent anion channel (VDAC) is a β-barrel channel of the mitochondrial outer membrane (MOM) that passively transports ions, metabolites, polypeptides, and single-stranded DNA. VDAC responds to a transmembrane potential by "gating," i.e. transitioning to one of a variety of low-conducting states of unknown structure. The gated state results in nearly complete suppression of multivalent mitochondrial metabolite (such as ATP and ADP) transport, while enhancing calcium transport. Voltage gating is a universal property of β-barrel channels, but VDAC gating is anomalously sensitive to transmembrane potential. Here, we show that a single residue in the pore interior, K12, is responsible for most of VDAC's voltage sensitivity. Using the analysis of over 40 μs of atomistic molecular dynamics (MD) simulations, we explore correlations between motions of charged residues inside the VDAC pore and geometric deformations of the β-barrel. Residue K12 is bistable; its motions between two widely separated positions along the pore axis enhance the fluctuations of the β-barrel and augment the likelihood of gating. Single channel electrophysiology of various K12 mutants reveals a dramatic reduction of the voltage-induced gating transitions. The crystal structure of the K12E mutant at a resolution of 2.6 Å indicates a similar architecture of the K12E mutant to the wild type; however, 60 μs of atomistic MD simulations using the K12E mutant show restricted motion of residue 12, due to enhanced connectivity with neighboring residues, and diminished amplitude of barrel motions. We conclude that β-barrel fluctuations, governed particularly by residue K12, drive VDAC gating transitions.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures




Similar articles
-
Insights into VDAC Gating: Room-Temperature X-ray Crystal Structure of mVDAC-1.Biomolecules. 2024 Sep 24;14(10):1203. doi: 10.3390/biom14101203. Biomolecules. 2024. PMID: 39456136 Free PMC article.
-
Is the Voltage-Dependent Anion Channel a Major Player in Neurodegenerative Diseases?Int J Mol Sci. 2025 Jun 26;26(13):6138. doi: 10.3390/ijms26136138. Int J Mol Sci. 2025. PMID: 40649921 Free PMC article. Review.
-
Photoaffinity labeling with a neuroactive steroid analogue. 6-azi-pregnanolone labels voltage-dependent anion channel-1 in rat brain.J Biol Chem. 2003 Apr 11;278(15):13196-206. doi: 10.1074/jbc.M213168200. Epub 2003 Jan 30. J Biol Chem. 2003. PMID: 12560326
-
Atomistic simulation of voltage activation of a truncated BK channel.Elife. 2025 Sep 8;14:RP105895. doi: 10.7554/eLife.105895. Elife. 2025. PMID: 40920564 Free PMC article.
-
Regulation of Mitochondrial Respiration by VDAC Is Enhanced by Membrane-Bound Inhibitors with Disordered Polyanionic C-Terminal Domains.Int J Mol Sci. 2021 Jul 8;22(14):7358. doi: 10.3390/ijms22147358. Int J Mol Sci. 2021. PMID: 34298976 Free PMC article. Review.
Cited by
-
Gating of β-Barrel Protein Pores, Porins, and Channels: An Old Problem with New Facets.Int J Mol Sci. 2023 Jul 28;24(15):12095. doi: 10.3390/ijms241512095. Int J Mol Sci. 2023. PMID: 37569469 Free PMC article. Review.
-
Conformational plasticity of mitochondrial VDAC2 controls the kinetics of its interaction with cytosolic proteins.Sci Adv. 2025 Apr 25;11(17):eadv4410. doi: 10.1126/sciadv.adv4410. Epub 2025 Apr 23. Sci Adv. 2025. PMID: 40267181 Free PMC article.
-
Beta-Barrel Channel Response to High Electric Fields: Functional Gating or Reversible Denaturation?Int J Mol Sci. 2023 Nov 23;24(23):16655. doi: 10.3390/ijms242316655. Int J Mol Sci. 2023. PMID: 38068977 Free PMC article.
-
Insights into VDAC Gating: Room-Temperature X-ray Crystal Structure of mVDAC-1.Biomolecules. 2024 Sep 24;14(10):1203. doi: 10.3390/biom14101203. Biomolecules. 2024. PMID: 39456136 Free PMC article.
-
Solute Transport through Mitochondrial Porins In Vitro and In Vivo.Biomolecules. 2024 Mar 4;14(3):303. doi: 10.3390/biom14030303. Biomolecules. 2024. PMID: 38540723 Free PMC article. Review.
References
-
- Colombini M VDAC: The channel at the interface between mitochondria and the cytosol. Mol. Cell. Biochem. 2004, 256 (1−2), 107–115. - PubMed
-
- Lemasters JJ; Holmuhamedov E Voltage-dependent anion channel (VDAC) as mitochondrial governator–thinking outside the box. Biochimica et biophysica acta 2006, 1762 (2), 181–90. - PubMed
-
- Colombini M Pore-Size and Properties of Channels from Mitochondria Isolated from Neurospora-Crassa. J. Membr. Biol. 1980, 53 (2), 79–84.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

