A circuit mechanism for independent modulation of excitatory and inhibitory firing rates after sensory deprivation
- PMID: 35925891
- PMCID: PMC9371725
- DOI: 10.1073/pnas.2116895119
A circuit mechanism for independent modulation of excitatory and inhibitory firing rates after sensory deprivation
Abstract
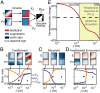
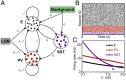
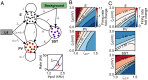
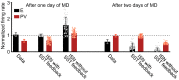
Diverse interneuron subtypes shape sensory processing in mature cortical circuits. During development, sensory deprivation evokes powerful synaptic plasticity that alters circuitry, but how different inhibitory subtypes modulate circuit dynamics in response to this plasticity remains unclear. We investigate how deprivation-induced synaptic changes affect excitatory and inhibitory firing rates in a microcircuit model of the sensory cortex with multiple interneuron subtypes. We find that with a single interneuron subtype (parvalbumin-expressing [PV]), excitatory and inhibitory firing rates can only be comodulated-increased or decreased together. To explain the experimentally observed independent modulation, whereby one firing rate increases and the other decreases, requires strong feedback from a second interneuron subtype (somatostatin-expressing [SST]). Our model applies to the visual and somatosensory cortex, suggesting a general mechanism across sensory cortices. Therefore, we provide a mechanistic explanation for the differential role of interneuron subtypes in regulating firing rates, contributing to the already diverse roles they serve in the cortex.
Keywords: cortical circuits; interneurons; network model; sensory deprivation; synaptic plasticity.
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
A layered microcircuit model of somatosensory cortex with three interneuron types and cell-type-specific short-term plasticity.Cereb Cortex. 2024 Sep 3;34(9):bhae378. doi: 10.1093/cercor/bhae378. Cereb Cortex. 2024. PMID: 39344196 Free PMC article.
-
Deciphering functional roles of synaptic plasticity and intrinsic neural firing in developing mouse visual cortex layer IV microcircuit.J Comput Neurosci. 2023 Feb;51(1):23-42. doi: 10.1007/s10827-022-00823-x. Epub 2022 Jun 23. J Comput Neurosci. 2023. PMID: 35737171
-
Disinhibitory Circuitry Gates Associative Synaptic Plasticity in Olfactory Cortex.J Neurosci. 2022 Apr 6;42(14):2942-2950. doi: 10.1523/JNEUROSCI.1369-21.2021. Epub 2022 Feb 18. J Neurosci. 2022. PMID: 35181596 Free PMC article.
-
Multiple shared mechanisms for homeostatic plasticity in rodent somatosensory and visual cortex.Philos Trans R Soc Lond B Biol Sci. 2017 Mar 5;372(1715):20160157. doi: 10.1098/rstb.2016.0157. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28093551 Free PMC article. Review.
-
Parvalbumin-Positive Interneurons Regulate Cortical Sensory Plasticity in Adulthood and Development Through Shared Mechanisms.Front Neural Circuits. 2022 May 6;16:886629. doi: 10.3389/fncir.2022.886629. eCollection 2022. Front Neural Circuits. 2022. PMID: 35601529 Free PMC article. Review.
Cited by
-
Spatiotemporal properties of cortical excitatory and inhibitory neuron activation by sustained and bursting electrical microstimulation.iScience. 2025 May 20;28(6):112707. doi: 10.1016/j.isci.2025.112707. eCollection 2025 Jun 20. iScience. 2025. PMID: 40520112 Free PMC article.
-
Chronic stress causes striatal disinhibition mediated by SOM-interneurons in male mice.Nat Commun. 2022 Nov 29;13(1):7355. doi: 10.1038/s41467-022-35028-4. Nat Commun. 2022. PMID: 36446783 Free PMC article.
-
Concurrent Encoding of Sequence Predictability and Event-Evoked Prediction Error in Unfolding Auditory Patterns.J Neurosci. 2024 Apr 3;44(14):e1894232024. doi: 10.1523/JNEUROSCI.1894-23.2024. J Neurosci. 2024. PMID: 38350998 Free PMC article.
-
Effects of stimulus pulse rate on somatosensory adaptation in the human cortex.Brain Stimul. 2022 Jul-Aug;15(4):987-995. doi: 10.1016/j.brs.2022.05.021. Epub 2022 Jun 4. Brain Stimul. 2022. PMID: 35671947 Free PMC article.
-
Top-down modulation in canonical cortical circuits with short-term plasticity.Proc Natl Acad Sci U S A. 2024 Apr 16;121(16):e2311040121. doi: 10.1073/pnas.2311040121. Epub 2024 Apr 9. Proc Natl Acad Sci U S A. 2024. PMID: 38593083 Free PMC article.
References
-
- Letzkus J. J., et al. ., A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480, 331–335 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

