Efficacy and safety of anakinra in adults presenting deteriorating respiratory symptoms from COVID-19: A randomized controlled trial
- PMID: 35925914
- PMCID: PMC9351999
- DOI: 10.1371/journal.pone.0269065
Efficacy and safety of anakinra in adults presenting deteriorating respiratory symptoms from COVID-19: A randomized controlled trial
Abstract
Objective: We aimed to investigate whether anakinra, an interleukin-1receptor inhibitor, could improve outcome in moderate COVID-19 patients.
Methods: In this controlled, open-label trial, we enrolled adults with COVID-19 requiring oxygen. We randomly assigned patients to receive intravenous anakinra plus optimized standard of care (oSOC) vs. oSOC alone. The primary outcome was treatment success at day 14 defined as patient alive and not requiring mechanical ventilation or extracorporeal membrane oxygenation.
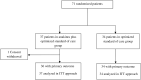
Results: Between 27th April and 6th October 2020, we enrolled 71 patients (240 patients planned to been enrolled): 37 were assigned to the anakinra group and 34 to oSOC group. The study ended prematurely by recommendation of the data and safety monitoring board due to safety concerns. On day 14, the proportion of treatment success was significantly lower in the anakinra group 70% (n = 26) vs. 91% (n = 31) in the oSOC group: risk difference-21 percentage points (95% CI, -39 to -2), odds ratio 0.23 (95% CI, 0.06 to 0.91), p = 0.027. After a 28-day follow-up, 9 patients in the anakinra group and 3 in the oSOC group had died. Overall survival at day 28 was 75% (95% CI, 62% to 91%) in the anakinra group versus 91% (95% CI, 82% to 100%) (p = 0.06) in the oSOC group. Serious adverse events occurred in 19 (51%) patients in the anakinra group and 18 (53%) in the oSOC group (p = 0·89).
Conclusion: This trial did not show efficacy of anakinra in patients with COVID-19. Furthermore, contrary to our hypothesis, we found that anakinra was inferior to oSOC in patients with moderate COVID-19 pneumonia.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N Engl J Med. July 17, 2020;NEJMoa2021436.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

