Pulmonary Surfactant Proteins Are Inhibited by Immunoglobulin A Autoantibodies in Severe COVID-19
- PMID: 35926164
- PMCID: PMC9952873
- DOI: 10.1164/rccm.202201-0011OC
Pulmonary Surfactant Proteins Are Inhibited by Immunoglobulin A Autoantibodies in Severe COVID-19
Abstract
Rationale: Coronavirus disease 2019 (COVID-19) can lead to acute respiratory distress syndrome with fatal outcomes. Evidence suggests that dysregulated immune responses, including autoimmunity, are key pathogenic factors. Objectives: To assess whether IgA autoantibodies target lung-specific proteins and contribute to disease severity. Methods: We collected 147 blood, 9 lung tissue, and 36 BAL fluid samples from three tertiary hospitals in Switzerland and one in Germany. Severe COVID-19 was defined by the need to administer oxygen. We investigated the presence of IgA autoantibodies and their effects on pulmonary surfactant in COVID-19 using the following methods: immunofluorescence on tissue samples, immunoprecipitations followed by mass spectrometry on BAL fluid samples, enzyme-linked immunosorbent assays on blood samples, and surface tension measurements with medical surfactant. Measurements and Main Results: IgA autoantibodies targeting pulmonary surfactant proteins B and C were elevated in patients with severe COVID-19 but not in patients with influenza or bacterial pneumonia. Notably, pulmonary surfactant failed to reduce surface tension after incubation with either plasma or purified IgA from patients with severe COVID-19. Conclusions: Our data suggest that patients with severe COVID-19 harbor IgA autoantibodies against pulmonary surfactant proteins B and C and that these autoantibodies block the function of lung surfactant, potentially contributing to alveolar collapse and poor oxygenation.
Keywords: COVID-19; autoimmunity; immunoglobulin A; pulmonary surfactant; pulmonary-associated surfactant protein.
Figures

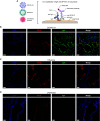
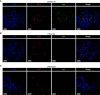


Comment in
-
Adding Insult to Injury: Does COVID-19 Promote Acute Respiratory Distress Syndrome by Inhibiting Surfactant?Am J Respir Crit Care Med. 2023 Jan 1;207(1):5-6. doi: 10.1164/rccm.202208-1549ED. Am J Respir Crit Care Med. 2023. PMID: 35976979 Free PMC article. No abstract available.
References
-
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA . 2020;324:782–793. - PubMed
-
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Oxford COVID Vaccine Trial Group Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet . 2021;397:99–111. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

