Programmable Bispecific Nano-immunoengager That Captures T Cells and Reprograms Tumor Microenvironment
- PMID: 35926215
- PMCID: PMC9479133
- DOI: 10.1021/acs.nanolett.2c00582
Programmable Bispecific Nano-immunoengager That Captures T Cells and Reprograms Tumor Microenvironment
Abstract
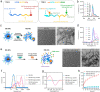
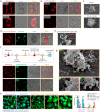
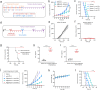
Immune checkpoint blockade (ICB) therapy has revolutionized clinical oncology. However, the efficacy of ICB therapy is limited by the ineffective infiltration of T effector (Teff) cells to tumors and the immunosuppressive tumor microenvironment (TME). Here, we report a programmable tumor cells/Teff cells bispecific nano-immunoengager (NIE) that can circumvent these limitations to improve ICB therapy. The peptidic nanoparticles (NIE-NPs) bind tumor cell surface α3β1 integrin and undergo in situ transformation into nanofibrillar network nanofibers (NIE-NFs). The prolonged retained nanofibrillar network at the TME captures Teff cells via the activatable α4β1 integrin ligand and allows sustained release of resiquimod for immunomodulation. This bispecific NIE eliminates syngeneic 4T1 breast cancer and Lewis lung cancer models in mice, when given together with anti-PD-1 antibody. The in vivo structural transformation-based supramolecular bispecific NIE represents an innovative class of programmable receptor-mediated targeted immunotherapeutics to greatly enhance ICB therapy against cancers.
Keywords: T cells capture; fibrillar transformation; immune checkpoint blockade (ICB) therapy; nano-immuno-engager.
Conflict of interest statement
The authors declare the following competing financial interest(s): K.S.L. and L.Z. are the coinventors of a patent on fibrillar transformable nanoparticles (PCT/US2020/046495). K.S.L. is the founding scientist of LamnoTherapeutics Inc., which plans to develop the nanotherapeutics described in the manuscript. The remaining authors declare no competing interests.
Figures