Synthesis of Vicinal Carbocycles by Intramolecular Nickel-Catalyzed Conjunctive Cross-Electrophile Coupling Reaction
- PMID: 35926218
- PMCID: PMC9396665
- DOI: 10.1021/acs.orglett.2c02481
Synthesis of Vicinal Carbocycles by Intramolecular Nickel-Catalyzed Conjunctive Cross-Electrophile Coupling Reaction
Abstract
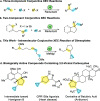
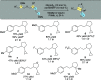
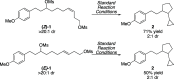
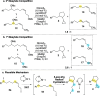
A nickel-catalyzed intramolecular conjunctive cross-electrophile coupling reaction has been established. This method enables the synthesis of 3,5-vicinal carbocyclic rings found in numerous biologically active compounds and natural products. We provide mechanistic experiments that indicate this reaction proceeds through alkyl iodides formed in situ, initiates at the secondary electrophilic center, and proceeds through radical intermediates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Nickel-Catalyzed Stereoselective Coupling Reactions of Benzylic and Alkyl Alcohol Derivatives.Acc Chem Res. 2023 Nov 21;56(22):3313-3324. doi: 10.1021/acs.accounts.3c00547. Epub 2023 Nov 7. Acc Chem Res. 2023. PMID: 37936256 Free PMC article.
-
Synthesis of Multisubstituted Allylic Alcohols via a Nickel-Catalyzed Cross-Electrophile Ring-Opening Reaction.Org Lett. 2022 Aug 12;24(31):5751-5755. doi: 10.1021/acs.orglett.2c02199. Epub 2022 Jul 28. Org Lett. 2022. PMID: 35901221
-
Nickel-Catalyzed Alkyl-Alkyl Cross-Electrophile Coupling Reaction of 1,3-Dimesylates for the Synthesis of Alkylcyclopropanes.J Am Chem Soc. 2020 Mar 18;142(11):5017-5023. doi: 10.1021/jacs.0c01330. Epub 2020 Mar 4. J Am Chem Soc. 2020. PMID: 32129601 Free PMC article.
-
A Unified Explanation for Chemoselectivity and Stereospecificity of Ni-Catalyzed Kumada and Cross-Electrophile Coupling Reactions of Benzylic Ethers: A Combined Computational and Experimental Study.J Am Chem Soc. 2019 Apr 10;141(14):5835-5855. doi: 10.1021/jacs.9b00097. Epub 2019 Mar 26. J Am Chem Soc. 2019. PMID: 30866626 Free PMC article.
-
Ni-Catalyzed C-C Couplings Using Alkyl Electrophiles.Top Curr Chem (Cham). 2016 Oct;374(5):66. doi: 10.1007/s41061-016-0067-6. Epub 2016 Aug 31. Top Curr Chem (Cham). 2016. PMID: 27580894 Review.
Cited by
-
Improved Process for the Synthesis of 3-(3-Trifluoromethylphenyl)propanal for More Sustainable Production of Cinacalcet HCl.Molecules. 2023 Aug 13;28(16):6042. doi: 10.3390/molecules28166042. Molecules. 2023. PMID: 37630295 Free PMC article.
-
Cross-Electrophile Coupling: Principles, Methods, and Applications in Synthesis.Chem Rev. 2024 Dec 11;124(23):13397-13569. doi: 10.1021/acs.chemrev.4c00524. Epub 2024 Nov 26. Chem Rev. 2024. PMID: 39591522 Free PMC article. Review.
References
-
-
Reviews of nickel catalysis:
- Modern Organonickel Chemistry; Tamaru Y., Ed.; Wiley-VCH: Weinheim, 2005.
- Tasker S. Z.; Standley E. A.; Jamison T. F. Recent Advances in Homogenous Nickel Catalysis. Nature 2014, 509, 299–309. 10.1038/nature13274. - DOI - PMC - PubMed
- Nickel Catalysis in Organic Synthesis: Methods and Reactions; Ogoshi S., Ed.; Wiley: 2020.
-
-
-
Reviews of conjunctive XC and XEC reactions:
- Derosa J.; Tran V. T.; van der Puyl V. A.; Engle K. M. Carbon–Carbon Bond π-Bonds as Conjunctive Reagents in Cross-Coupling. Aldrichimica Acta 2018, 51, 21–32.
- Zhang J.-S.; Liu L.; Chen T.; Han L.-B. Transition-Metal-Catalyzed Three-Component Difunctionalization of Alkenes. Chem.—Asian J. 2018, 13, 2277–2291. 10.1002/asia.201800647. - DOI - PubMed
- Qi X.; Diao T. Nickel-Catalyzed Dicarbofunctionalization of Alkenes. ACS Catal. 2020, 10, 8542–8556. 10.1021/acscatal.0c02115. - DOI - PMC - PubMed
- Derosa J.; Apolinar O.; Kang T.; Tran V. T.; Engle K. M. Recent Deveopments in Nickel-Catalyzed Intermolecular Dicarbofunctionalization of Alkenes. Chem. Sci. 2020, 11, 4287–4296. 10.1039/C9SC06006E. - DOI - PMC - PubMed
- Badir S. O.; Molander G. A. Developments in Photoredox/Nickel Dual Catalyzed 1,2-Dicarbofunctionalizations. Chem. 2020, 6, 1327–1339. 10.1016/j.chempr.2020.05.013. - DOI - PMC - PubMed
- Tu H.-Y.; Zhu S.; Qing F.-L.; Chu L. Recent Advances in Nickel-Catalyzed Three Component Difunctionalization of Unactivated Alkenes. Synthesis 2020, 52, 1346–1356. 10.1055/s-0039-1690842. - DOI
- Ping Y.; Kong W. Ni-Catalyzed Reductive Difunctionalization of Alkenes. Synthesis 2020, 52, 979–992. 10.1055/s-0039-1690807. - DOI
- Wickham L. M.; Giri R. Transition Metal (Ni, Cu, Pd)-Catalyzed Alkene Dicarbofunctionalization Reactions. Acc. Chem. Res. 2021, 54, 3415–3437. 10.1021/acs.accounts.1c00329. - DOI - PMC - PubMed
- Zhu S.; Zhao X.; Li H.; Chu L. Catalytic Three-Component Dicarbofuncationalization Reaction Involving Radical Capture by Nickel. Chem. Soc. Rev. 2021, 50, 10836–10856. 10.1039/D1CS00399B. - DOI - PubMed
- Gao P.; Niu Y.-J.; Yang F.; Guo L. N.; Duan X.-H. Three-Component 1,2-Dicarbofunctionalization of Alkenes Involving Alkyl Radicals. Chem. Commun. 2022, 58, 730–746. 10.1039/D1CC05730H. - DOI - PubMed
-
-
-
Reviews of XEC reactions:
- Knappke C. E. I.; Grupe S.; Gärtner D.; Corpet M.; Gosmini C.; Jacobi von Wangelin A. Reductive Cross-Coupling Reactions between Two Electrophiles. Chem.—Eur. J. 2014, 20, 6828–6842. 10.1002/chem.201402302. - DOI - PubMed
- Everson D. A.; Weix D. J. Cross-Electrophile Coupling: Principles of Reactivity and Selectivity. J. Org. Chem. 2014, 79, 4793–4798. 10.1021/jo500507s. - DOI - PMC - PubMed
- Gu J.; Wang X.; Xue W.; Gong H. Nickel-Catalyzed Reductive Coupling of Alkyl Halides with Other Electrophiles: Concept and Mechanistic Considerations. Org. Chem. Front. 2015, 2, 1411–1421. 10.1039/C5QO00224A. - DOI
- Goldfogel M.; Huang L.; Weix D. J.. Cross-Electrophile Coupling. In Nickel Catalysis in Organic Synthesis; Ogoshi S., Ed.; Wiley, 2020; 183–222.
- Richmond E.; Moran J. Recent Advances in Nickel Catalysis Enabled by Stoichiometric Metallic Reducing Agents. Synthesis 2018, 50, 499–513. 10.1055/s-0036-1591853. - DOI
- Campeau L.-C.; Hazari N. Cross-Coupling and Related Reactions: Connecting Past Success to the Development of New Reactions for the Future. Organometallics 2019, 38, 3–35. 10.1021/acs.organomet.8b00720. - DOI - PMC - PubMed
- Hewitt K. A.; Lin P. C.; Raffman E. T. A.; Jarvo E. R.. C–C Bond Formation Through Cross-Electrophile Coupling Reaction. Comprehensive Organometallic Chemistry IV; Elsevier: 2021.
-
-
-
Discussion of conjunctive XEC reaction mechanisms:
- Lin Q.; Diao T. Mechanism of Ni-Catalyzed Reductive 1,2-Dicarbofunctionalization of Alkenes. J. Am. Chem. Soc. 2019, 141, 17937–17948. 10.1021/jacs.9b10026. - DOI - PMC - PubMed
- Diccianni J.; Lin Q.; Diao T. Mechanism of Nickel-Catalyzed Coupling Reactions and Applications in Alkene Functionalization. Acc. Chem. Res. 2020, 53, 906–919. 10.1021/acs.accounts.0c00032. - DOI - PMC - PubMed
-
-
-
Representative examples of conjunctive XEC reactions using activated alkenes:
- García-Domínguez A.; Li Z.; Nevado C. Nickel-Catalyzed Reductive Dicarbofunctionalization of Alkenes. J. Am. Chem. Soc. 2017, 139, 6835–6838. 10.1021/jacs.7b03195. - DOI - PubMed
- Wang L.; Wang C. Nickel-Catalyzed Three-Component Reductive Alkylacylation of Electron Deficient Activated Alkenes. Org. Lett. 2020, 22, 8829–8835. 10.1021/acs.orglett.0c03210. - DOI - PubMed
- Zhao Q.-W.; Yang Z.-F.; Fu X.-P.; Zhang X. Access to α,α-Diflouro-γ-Amino Acids by Nickel-Catalyzed Reductive Aryldifluoroacetylation of N-Vinylacetamide. Synlett 2021, 32, 1565–1569. 10.1055/s-0040-1706553. - DOI
- Wang X.-X.; Lu X.; He S.-J.; Fu Y. Nickel-Catalyzed Three-Component Olefin Reductive Dicarbofunctionalization to Access Alkylborates. Chem. Sci. 2020, 11, 7950–7956. 10.1039/D0SC02054K. - DOI - PMC - PubMed
- Feng X.; Guo L.; Zhu S.; Chu L. Borates as a Traceless Activation Group for Intermolecular Alkylarylation of Ethylene through Photoredox/Nickel Dual Catalysis. Synlett 2021, 32, 1519–1524. 10.1055/a-1320-6946. - DOI
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

