Adenosine metabolized from extracellular ATP promotes type 2 immunity through triggering A2BAR signaling in intestinal epithelial cells
- PMID: 35926464
- PMCID: PMC9402265
- DOI: 10.1016/j.celrep.2022.111150
Adenosine metabolized from extracellular ATP promotes type 2 immunity through triggering A2BAR signaling in intestinal epithelial cells
Abstract
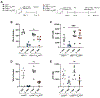
Intestinal nematode parasites can cross the epithelial barrier, causing tissue damage and release of danger-associated molecular patterns (DAMPs) that may promote host protective type 2 immunity. We investigate whether adenosine binding to the A2B adenosine receptor (A2BAR) on intestinal epithelial cells (IECs) plays an important role. Specific blockade of IEC A2BAR inhibits the host protective memory response to the enteric helminth, Heligmosomoides polygyrus bakeri (Hpb), including disruption of granuloma development at the host-parasite interface. Memory T cell development is blocked during the primary response, and transcriptional analyses reveal profound impairment of IEC activation. Extracellular ATP is visualized 24 h after inoculation and is shown in CD39-deficient mice to be critical for the adenosine production mediating the initiation of type 2 immunity. Our studies indicate a potent adenosine-mediated IEC pathway that, along with the tuft cell circuit, is critical for the activation of type 2 immunity.
Keywords: CP: Immunology; CP: Molecular biology.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests G.H. owns stock in Purine Pharmaceuticals and has patents related to purinergic signaling in sepsis. S.C.R. is the scientific cofounder of Purinomia and a consultant to SynLogic and eGenesis.
Figures





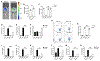
References
-
- Antonioli L, Fornai M, Colucci R, Ghisu N, Tuccori M, Del Tacca M, and Blandizzi C (2008). Regulation of enteric functions by adenosine: pathophysiological and pharmacological implications. Pharmacol. Ther 120, 233–253. - PubMed
-
- Antonioli L, Blandizzi C, Pacher P, and Haskó G (2013a). Immunity, inflammation and cancer: a leading role for adenosine. Nat. Rev. Cancer 13, 842–857. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

