Conversion of anterograde into retrograde trains is an intrinsic property of intraflagellar transport
- PMID: 35926510
- PMCID: PMC9521741
- DOI: 10.1016/j.cub.2022.07.033
Conversion of anterograde into retrograde trains is an intrinsic property of intraflagellar transport
Abstract
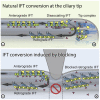
Cilia or eukaryotic flagella are microtubule-based organelles found across the eukaryotic tree of life. Their very high aspect ratio and crowded interior are unfavorable to diffusive transport of most components required for their assembly and maintenance. Instead, a system of intraflagellar transport (IFT) trains moves cargo rapidly up and down the cilium (Figure 1A).1-3 Anterograde IFT, from the cell body to the ciliary tip, is driven by kinesin-II motors, whereas retrograde IFT is powered by cytoplasmic dynein-1b motors.4 Both motors are associated with long chains of IFT protein complexes, known as IFT trains, and their cargoes.5-8 The conversion from anterograde to retrograde motility at the ciliary tip involves (1) the dissociation of kinesin motors from trains,9 (2) a fundamental restructuring of the train from the anterograde to the retrograde architecture,8,10,11 (3) the unloading and reloading of cargo,2 and (4) the activation of the dynein motors.8,12 A prominent hypothesis is that there is dedicated calcium-dependent protein-based machinery at the ciliary tip to mediate these processes.4,13 However, the mechanisms of IFT turnaround have remained elusive. In this study, we use mechanical and chemical methods to block IFT at intermediate positions along the cilia of the green algae Chlamydomonas reinhardtii, in normal and calcium-depleted conditions. We show that IFT turnaround, kinesin dissociation, and dynein-1b activation can consistently be induced at arbitrary distances from the ciliary tip, with no stationary tip machinery being required. Instead, we demonstrate that the anterograde-to-retrograde conversion is a calcium-independent intrinsic ability of IFT.
Keywords: TIRF microscopy; cilia and flagella; ciliary tip; intraflagellar transport; micromanipulator; total-internal reflection microscopy.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



Comment in
-
Intraflagellar transport: Derailing causes turnarounds.Curr Biol. 2022 Sep 26;32(18):R967-R969. doi: 10.1016/j.cub.2022.07.061. Curr Biol. 2022. PMID: 36167049
References
-
- Picariello T., Brown J.M., Hou Y., Swank G., Cochran D.A., King O.D., Lechtreck K., Pazour G.J., Witman G.B. A global analysis of IFT-A function reveals specialization for transport of membrane-associated proteins into cilia. J. Cell Sci. 2019;132:jcs220749. doi: 10.1242/jcs.220749. - DOI - PMC - PubMed
-
- Cole D.G., Diener D.R., Himelblau A.L., Beech P.L., Fuster J.C., Rosenbaum J.L. Chlamydomonas Kinesin-II-dependent Intraflagellar Transport (IFT): IFT Particles Contain Proteins Required for Ciliary Assembly in Caenorhabditis elegans Sensory Neurons. J. Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

