Pre-exposure prophylaxis with tixagevimab and cilgavimab (Evusheld) for COVID-19 among 1112 severely immunocompromised patients
- PMID: 35926762
- PMCID: PMC9340091
- DOI: 10.1016/j.cmi.2022.07.015
Pre-exposure prophylaxis with tixagevimab and cilgavimab (Evusheld) for COVID-19 among 1112 severely immunocompromised patients
Abstract
Objective: Immunocompromised patients have an increased risk of a severe form of COVID-19. The clinical efficacy of the tixagevimab/cilgavimab monoclonal antibody combination as pre-exposure prophylaxis against BA.1 and BA.2 SARS-CoV-2 Omicron sublineages is unknown. We aimed to describe the incidence and outcomes of COVID-19 among immunocompromised patients receiving tixagevimab/cilgavimab as preexposure prophylaxis during the Omicron wave in France.
Methods: This was an observational multicentre cohort study of immunocompromised patients receiving tixagevimab/cilgavimab as preexposure prophylaxis between December 28, 2021 and March 31, 2022. Patients received tixagevimab/cilgavimab 150/150 mg intramuscularly if they had impaired vaccine response and a high risk of severe form of COVID-19.
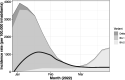
Results: Tixagevimab/cilgavimab was administered to 1112 immunocompromised patients. After a median (range) follow-up of 63 (49-73) days, COVID-19 was confirmed in 49/1112 (4.4%) ≥5 days after treatment. During the study period, mean weekly incidence rate was 1669 in 100 000 inhabitants in Ile-de-France and 530 in 100 000 among patients who received tixagevimab/cilgavimab prophylaxis. Among infected patients, 43/49 (88%) had a mild-to-moderate form and 6/49 (12%) had a moderate-to-severe form of COVID-19. Patients with moderate-to-severe illnesses were less likely to have received early therapies than patients with mild forms (53.5% vs. 16.7% respectively) and 2/49 (4%) patients died from COVID-19.
Discussion: Our study reported a low rate of infections and severe illnesses among immunocompromised patients treated with tixagevimab/cilgavimab. A global preventive strategy including vaccines, preexposure prophylaxis with monoclonal antibodies, and early therapies might be effective to prevent severe forms of COVID-19 among severely immunocompromised patients.
Keywords: COVID-19; Cilgavimab; Immunocompromised; Monoclonal antibodies; Preexposure prophylaxis; SARS-CoV-2; Tixagevimab.
Copyright © 2022 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures
References
-
- Bruel T., Hadjadj J., Maes P., Planas D., Seve A., Staropoli I., et al. Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies. Nat Med. 2022;28:1297–1302. - PubMed
-
- Wise J. Covid-19: Evusheld is approved in UK for prophylaxis in immunocompromised people. BMJ. 2022;376:o722. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

