Intra-arterial thrombolytics during endovascular thrombectomy for acute ischaemic stroke in the MR CLEAN Registry
- PMID: 35926984
- PMCID: PMC9985803
- DOI: 10.1136/svn-2022-001677
Intra-arterial thrombolytics during endovascular thrombectomy for acute ischaemic stroke in the MR CLEAN Registry
Abstract
Introduction: The efficacy and safety of local intra-arterial (IA) thrombolytics during endovascular thrombectomy (EVT) for large-vessel occlusions is uncertain. We analysed how often IA thrombolytics were administered in the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN) Registry, whether it was associated with improved functional outcome and assessed technical and safety outcomes compared with EVT without IA thrombolytics.
Methods: In this observational study, we included patients undergoing EVT for an acute ischaemic stroke in the anterior circulation from the MR CLEAN Registry (March 2014-November 2017). The primary endpoint was favourable functional outcome, defined as an modified Rankin Scale score ≤2 at 90 days. Secondary endpoints were reperfusion status, early neurological recovery and symptomatic intracranial haemorrhage (sICH). Subgroup analyses for IA thrombolytics as primary versus adjuvant revascularisation attempt were performed.
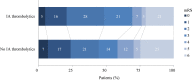
Results: Of the 2263 included patients, 95 (4.2%) received IA thrombolytics during EVT. The IA thrombolytics administered were urokinase (median dose, 250 000 IU (IQR, 1 93 750-2 50 000)) or alteplase (median dose, 20 mg (IQR, 12-20)). No association was found between IA thrombolytics and favourable functional outcome (adjusted OR (aOR), 1.16; 95% CI 0.71 to 1.90). Successful reperfusion was less often observed in those patients treated with IA thrombolytics (aOR, 0.57; 95% CI 0.36 to 0.90). The odds of sICH (aOR, 0.82; 95% CI 0.32 to 2.10) and early neurological recovery were comparable between patients treated with and without IA thrombolytics. For primary and adjuvant revascularisation attempts, IA thrombolytics were more often administered for proximal than for distal occlusions. Functional outcomes were comparable for patients receiving IA thrombolytics as a primary versus adjuvant revascularisation attempt.
Conclusion: Local IA thrombolytics were rarely used in the MR CLEAN Registry. In the relatively small study sample, no statistical difference was observed between groups in the rate of favourable functional outcome or sICH. Patients whom required and underwent IA thrombolytics were patients less likely to achieve successful reperfusion, probably due to selection bias.
Keywords: Stroke; Thrombectomy; Thrombolytic Therapy.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Amsterdam University Medical Centre received funds from Stryker® for consultations by Prof. Dr. Majoie, Prof. Dr. Roos, and Drs. Berkhemer. Maastricht University Medical Centre received funds from Stryker® and Codman® for consultations by Prof. Dr. Van Zwam. Prof. Dr. Majoie: Related: Grants TWIN Foundation; Unrelated: Grants from CVON/Dutch Heart Foundation, Stryker, European Commission, TWIN Foundation, Health Evaluation Netherlands (all paid to institution); shareholder of Nico-lab, a company that focuses on the use of artificial intelligence for medical image analysis (modest). The other authors report no conflicts.
Figures
References
-
- Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European cooperative acute stroke study (ECASS). JAMA 1995;274:1017–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
