Recent advances in clinical practice: advances in cross-sectional imaging in inflammatory bowel disease
- PMID: 35927032
- PMCID: PMC9664122
- DOI: 10.1136/gutjnl-2021-326562
Recent advances in clinical practice: advances in cross-sectional imaging in inflammatory bowel disease
Abstract
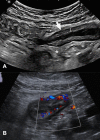
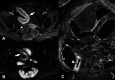
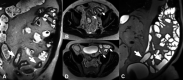
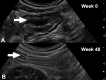
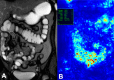
Endoscopy remains the reference standard for the diagnosis and assessment of patients with inflammatory bowel disease (IBD), but it has several important limitations. Cross-sectional imaging techniques such as magnetic resonance enterography (MRE) and intestinal ultrasound (IUS) are better tolerated and safer. Moreover, they can examine the entire bowel, even in patients with stenoses and/or severe inflammation. A variety of cross-sectional imaging activity scores strongly correlate with endoscopic measures of mucosal inflammation in the colon and terminal ileum. Unlike endoscopy, cross-sectional techniques allow complete visualisation of the small-bowel and assess for extraintestinal disease, which occurs in nearly half of patients with IBD. Extramural findings may predict outcomes better than endoscopic mucosal assessment, so cross-sectional techniques might help identify more relevant therapeutic targets. Coupled with their high sensitivity, these advantages have made MRE and IUS the primary non-invasive options for diagnosing and monitoring Crohn's disease; they are appropriate first-line investigations, and have become viable alternatives to colonoscopy. This review discusses cross-sectional imaging in IBD in current clinical practice as well as research lines that will define the future role of these techniques.
Keywords: CROHN'S DISEASE; GASTROINTESTINAL ULTRASOUND; INFLAMMATORY BOWEL DISEASE; MAGNETIC RESONANCE IMAGING.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JR has received grants from AbbVie and consulting fees from Boehringer Ingelheim, Janssen, Origo, Lument, Takeda and Alimentiv. JT has received grants from AbbVie and Janssen, advisory board fees and/or speaker fees from Janssen, Galapagos and Pfizer. SK has no declarations. SAT is a previous research consultant to Alimentiv. Shareholder in Motilent. TK has received grants from AbbVie, Janssen and Takeda, advisory board fees and/or speaker fees from AbbVie, Amgen, Arena Pharmaceuticals, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Dr Falk Pharma, Ferring Arzneimittel, Galapagos, Gilead, Janssen, MSD Sharp & amp; Dome GmbH, Pfizer, Roche, Takeda Pharma and Vifor Pharma.
Figures

References
-
- Turner D, Ricciuto A, Lewis A, et al. . STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International organization for the study of IBD (IOIBD): determining therapeutic goals for Treat-to-Target strategies in IBD. Gastroenterology 2021;160:1570–83. 10.1053/j.gastro.2020.12.031 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
