Engineering human JMJD2A tudor domains for an improved understanding of histone peptide recognition
- PMID: 35927178
- PMCID: PMC9771871
- DOI: 10.1002/prot.26408
Engineering human JMJD2A tudor domains for an improved understanding of histone peptide recognition
Abstract
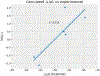
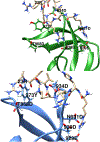
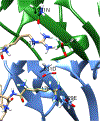
JMJD2A is a histone lysine demethylase which recognizes and demethylates H3K9me3 and H3K36me3 residues and is overexpressed in various cancers. It utilizes a tandem tudor domain to facilitate its own recruitment to histone sites, recognizing various di- and tri-methyl lysine residues with moderate affinity. In this study, we successfully engineered the tudor domain of JMJD2A to specifically bind to H4K20me3 with a 20-fold increase of affinity and improved selectivity. To reveal the molecular basis, we performed molecular dynamics and free energy decomposition analysis on the human JMJD2A tandem tudor domains bound to H4K20me2, H4K20me3, and H3K23me3 peptides to uncover the residues and conformational changes important for the enhanced binding affinity and selectivity toward H4K20me2/3. These analyses revealed new insights into understanding chromatin reader domains recognizing histone modifications and improving binding affinity and selectivity of these domains. Furthermore, we showed that the tight binding of JMJD2A to H4K20me2/3 is not sufficient to improve the efficiency of CRISPR-CAS9 mediated homology directed repair (HDR), suggesting a complicated relationship between JMJD2A and the DNA damage response beyond binding affinity toward the H4K20me2/3 mark.
Keywords: CRISPR; JMJD2A; epigenetics; histone; molecular dynamics.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
Figures


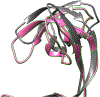

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

