Chronic nicotinamide mononucleotide supplementation elevates blood nicotinamide adenine dinucleotide levels and alters muscle function in healthy older men
- PMID: 35927255
- PMCID: PMC9158788
- DOI: 10.1038/s41514-022-00084-z
Chronic nicotinamide mononucleotide supplementation elevates blood nicotinamide adenine dinucleotide levels and alters muscle function in healthy older men
Abstract
Preclinical studies have revealed that the elevation of nicotinamide adenine dinucleotide (NAD + ) upon the administration of nicotinamide mononucleotide (NMN), an NAD + precursor, can mitigate aging-related disorders; however, human data on this are limited. We investigated whether the chronic oral supplementation of NMN can elevate blood NAD + levels and alter physiological dysfunctions in healthy older participants. We administered 250 mg NMN per day to aged men for 6 or 12 weeks in a placebo-controlled, randomized, double-blind, parallel-group trial. Chronic NMN supplementation was well tolerated and caused no significant deleterious effect. Metabolomic analysis of whole blood samples demonstrated that oral NMN supplementation significantly increased the NAD + and NAD + metabolite concentrations. There were nominally significant improvements in gait speed and performance in the left grip test, which should be validated in larger studies; however, NMN exerted no significant effect on body composition. Therefore, chronic oral NMN supplementation can be an efficient NAD + booster for preventing aging-related muscle dysfunctions in humans.
© 2022. The Author(s).
Conflict of interest statement
The authors Y.F., T. Sato, and T. Sakurai declare no competing non-financial interests, but do declare the following competing financial interests: they are employees of Mitsubishi Corporation Life Sciences Limited. The remaining authors declare no competing interests.
Figures
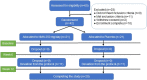

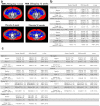
References
-
- Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr. Biol. 2012;22:R741–R752. - PubMed
LinkOut - more resources
Full Text Sources

