Pyrroloquinoline quinone (PQQ) protects mitochondrial function of HEI-OC1 cells under premature senescence
- PMID: 35927260
- PMCID: PMC9158787
- DOI: 10.1038/s41514-022-00083-0
Pyrroloquinoline quinone (PQQ) protects mitochondrial function of HEI-OC1 cells under premature senescence
Erratum in
-
Author Correction: Pyrroloquinoline quinone (PQQ) protects mitochondrial function of HEI-OC1 cells under premature senescence.NPJ Aging. 2022 Oct 6;8(1):14. doi: 10.1038/s41514-022-00095-w. NPJ Aging. 2022. PMID: 36202820 Free PMC article. No abstract available.
Abstract
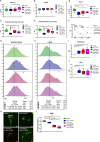
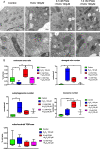
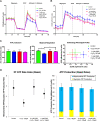
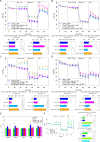
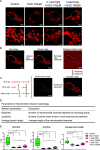
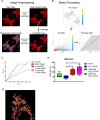
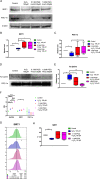
The aim of this study was to investigate the effects of pyrroloquinoline quinone (PQQ), an oxidoreductase cofactor, on the H2O2-induced premature senescence model in HEI-OC1 auditory cells and to elucidate its mechanism of action in vitro. Cells were treated with PQQ for 1 day before H2O2 (100 μM) exposure. Mitochondrial respiratory capacity was damaged in this premature senescence model but was restored in cells pretreated with PQQ (0.1 nM or 1.0 nM). A decrease in mitochondrial potential, the promotion of mitochondrial fusion and the accelerated movement of mitochondria were all observed in PQQ-pretreated cells. The protein expression of sirtuin 1 (SIRT1) and peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) were significantly decreased under H2O2 exposure while they were increased with PQQ pretreatment, and PGC-1α acetylation was significantly decreased. In conclusion, PQQ has a protective effect on the premature senescence model of HEI-OC1 auditory cells and is associated with the SIRT1/PGC-1α signaling pathway, mitochondrial structure, and mitochondrial respiratory capacity.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Pyrroloquinoline Quinone, a Redox-Active o-Quinone, Stimulates Mitochondrial Biogenesis by Activating the SIRT1/PGC-1α Signaling Pathway.Biochemistry. 2017 Dec 19;56(50):6615-6625. doi: 10.1021/acs.biochem.7b01185. Epub 2017 Dec 6. Biochemistry. 2017. PMID: 29185343
-
Pyrroloquinoline quinone stimulates mitochondrial biogenesis through cAMP response element-binding protein phosphorylation and increased PGC-1alpha expression.J Biol Chem. 2010 Jan 1;285(1):142-52. doi: 10.1074/jbc.M109.030130. Epub 2009 Oct 27. J Biol Chem. 2010. PMID: 19861415 Free PMC article.
-
Pyrroloquinoline Quinone Resists Denervation-Induced Skeletal Muscle Atrophy by Activating PGC-1α and Integrating Mitochondrial Electron Transport Chain Complexes.PLoS One. 2015 Dec 8;10(12):e0143600. doi: 10.1371/journal.pone.0143600. eCollection 2015. PLoS One. 2015. PMID: 26646764 Free PMC article.
-
PGC-1α Protects RPE Cells of the Aging Retina against Oxidative Stress-Induced Degeneration through the Regulation of Senescence and Mitochondrial Quality Control. The Significance for AMD Pathogenesis.Int J Mol Sci. 2018 Aug 7;19(8):2317. doi: 10.3390/ijms19082317. Int J Mol Sci. 2018. PMID: 30087287 Free PMC article. Review.
-
Pyrroloquinoline-Quinone Is More Than an Antioxidant: A Vitamin-like Accessory Factor Important in Health and Disease Prevention.Biomolecules. 2021 Sep 30;11(10):1441. doi: 10.3390/biom11101441. Biomolecules. 2021. PMID: 34680074 Free PMC article. Review.
Cited by
-
The efferocytosis process in aging: Supporting evidence, mechanisms, and therapeutic prospects for age-related diseases.J Adv Res. 2025 Mar;69:31-49. doi: 10.1016/j.jare.2024.03.008. Epub 2024 Mar 17. J Adv Res. 2025. PMID: 38499245 Free PMC article. Review.
-
Decreasing Intracellular Entropy by Increasing Mitochondrial Efficiency and Reducing ROS Formation-The Effect on the Ageing Process and Age-Related Damage.Int J Mol Sci. 2024 Jun 7;25(12):6321. doi: 10.3390/ijms25126321. Int J Mol Sci. 2024. PMID: 38928027 Free PMC article. Review.
-
Vaxtherapy, a Multiphase Therapeutic Protocol Approach for Longvax, the COVID-19 Vaccine-Induced Disease: Spike Persistence as the Core Culprit and Its Downstream Effects.Diseases. 2025 Jun 30;13(7):204. doi: 10.3390/diseases13070204. Diseases. 2025. PMID: 40709992 Free PMC article.
-
Premature senescence is regulated by crosstalk among TFEB, the autophagy lysosomal pathway and ROS derived from damaged mitochondria in NaAsO2-exposed auditory cells.Cell Death Discov. 2024 Aug 28;10(1):382. doi: 10.1038/s41420-024-02139-4. Cell Death Discov. 2024. PMID: 39191766 Free PMC article.
-
Current study on Pyrroloquinoline quinone (PQQ) therapeutic role in neurodegenerative diseases.Mol Biol Rep. 2025 Apr 15;52(1):397. doi: 10.1007/s11033-025-10491-6. Mol Biol Rep. 2025. PMID: 40234255 Review.
References
-
- Wang Z, Wei D, Xiao H. Methods of cellular senescence induction using oxidative stress. Methods Mol. Biol. 2013;1048:135–144. - PubMed
-
- Zdanov S, Remacle J, Toussaint O. Establishment of H2O2-induced premature senescence in human fibroblasts concomitant with increased cellular production of H2O2. Annal. New York Acad. Sci. 2006;1067:210–216. - PubMed
LinkOut - more resources
Full Text Sources

