Cingulate-motor circuits update rule representations for sequential choice decisions
- PMID: 35927275
- PMCID: PMC9352796
- DOI: 10.1038/s41467-022-32142-1
Cingulate-motor circuits update rule representations for sequential choice decisions
Abstract
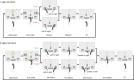
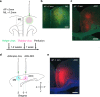
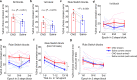
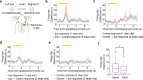
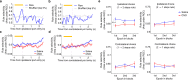
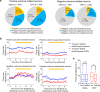
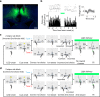
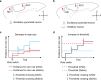
Anterior cingulate cortex mediates the flexible updating of an animal's choice responses upon rule changes in the environment. However, how anterior cingulate cortex entrains motor cortex to reorganize rule representations and generate required motor outputs remains unclear. Here, we demonstrate that chemogenetic silencing of the terminal projections of cingulate cortical neurons in secondary motor cortex in the rat disrupts choice performance in trials immediately following rule switches, suggesting that these inputs are necessary to update rule representations for choice decisions stored in the motor cortex. Indeed, the silencing of cingulate cortex decreases rule selectivity of secondary motor cortical neurons. Furthermore, optogenetic silencing of cingulate cortical neurons that is temporally targeted to error trials immediately after rule switches exacerbates errors in the following trials. These results suggest that cingulate cortex monitors behavioral errors and updates rule representations in motor cortex, revealing a critical role for cingulate-motor circuits in adaptive choice behaviors.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Lashley, K. S. The problem of serial order in behavior. In Cerebral Mechanisms in Behavior (ed. Jeffress, L. A.) 112–146 (Wiley, New York, 1951).
-
- Evarts, E. V., Shinoda, Y. & Wise, S.P. Neurophysiological Approaches to Higher Brain Functions (Wiley, New York, 1984).
-
- Passingham, R. The Frontal Lobes and Voluntary Action (Oxford University Press, Oxford, 1993).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

